Abstract
Urinary incontinence management varies depending on the type of incontinence and severity of symptoms. Types of incontinence include stress (SUI), urge or overactive bladder (OAB), mixed, neurogenic, and overflow incontinence. First-line treatment for OAB and SUI is nonpharmacologic management. Behavioral therapy is first-line treatment for urge incontinence. Vaginal mechanical devices (cones, pessaries, and urethral plugs), pelvic floor muscle training, and electroacupuncture are recommended as first-line treatment for women with SUI. Biofeedback and electric muscle stimulation can be adjunctive therapy for SUI. Antimuscarinics and β-3 agonists can be used as adjective therapy for those with OAB who do not improve with behavioral therapy. β-3 agonists have less anticholinergic side effects compared with antimuscarinics for OAB. Adverse medication effects can often lead to discontinuation due to poor tolerability. Third-line therapies are for those who fail conservative and pharmacologic therapies and lack high-grade evidence. Neuromodulation, neurotoxin injections, vaginal laser therapy, and acupuncture are third-line in OAB management. Pharmacologic management with α-1-blockers is recommended as first-line treatment for moderate to severe overflow incontinence from BPH. 5-α reductase inhibitors can be used as an adjunct medication in those with refractory overflow incontinence symptoms and a PSA ≥ 1.5 mg/dL. Clean intermittent catheterization is first-line therapy for neurogenic bladder but can increase risk of catheter-associated urinary tract infection. Clinicians should assess type of incontinence, patient goals, side effect profile, and tolerability to determine an individualized treatment plan for each patient.
- Family Medicine
- Overactive bladder
- Stress Urinary Incontinence
- Urge Urinary Incontinence
- Urinary Incontinence
Practice Recommendations
Behavioral therapy is first-line treatment for urge incontinence (SOR B)
Pelvic floor muscle training (PFMT) is recommended as first-line treatment for women with stress or mixed urinary incontinence, but there is no consensus about the most effective PFMT intervention due to heterogeneous data (SOR A)
α-1-blocker therapy is first-line therapy for overflow incontinence due to benign prostatic hyperplasia (BPH) (SOR A) followed by 5-α reductase inhibitors (SOR B)
Clean intermittent catheterization (CIC) is first-line therapy for neurogenic bladder, but can increase risk of catheter-associated urinary tract infection (CAUTI) (SOR C)
Introduction
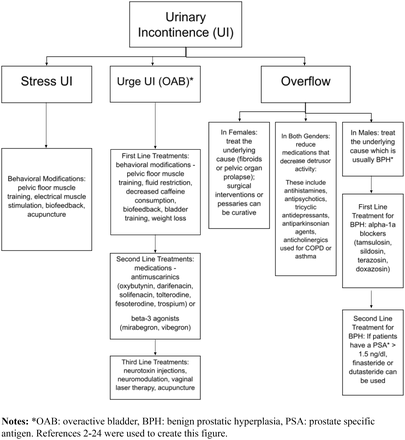
Urinary incontinence affects about 13 million adults in the United States and is often underreported due to the disconcerting nature of symptoms.1 Types of incontinence include stress (SUI), urge or overactive bladder (OAB), mixed, neurogenic, and overflow incontinence. See Table 1 for definitions. The prevalence of urinary incontinence varies with SUI affecting up to 45% of women over 30 years old, urge incontinence affecting 9% in women 40 to 44 years; 31% in women over 75 years, and 42% in men over 75 years.1 Mixed incontinence affects 20 to 30% of those with chronic incontinence, and overflow incontinence affects 5% of those with chronic incontinence.1 This article will focus on management of different types of urinary incontinence through nonoperative therapies (Figure 1).
Flowchart of types of urinary incontinence and corresponding treatments.
Types of Incontinence
Nonpharmacologic Management for OAB
Nonpharmacologic therapies can be beneficial for OAB as first-line or adjunctive therapy or for those who develop adverse effects with drugs.
Behavioral Therapies
As first-line or adjunctive therapy in management of OAB and SUI, behavioral therapies include fluid restriction (reduction by 25%), decreased caffeine intake, bladder-sphincter biofeedback (BF), bladder training, and pelvic floor muscle training (PFMT). Weight loss of 8% in obese women reduced UI episodes by 47% vs 28% in control groups.2 Per American Urologic Association (AUA) guidelines, behavioral therapy should be offered to all patients with OAB.2 After 6 weeks, behavioral therapy alone reduced incontinence episodes per week by 52.9% (from 6.8 to 3.1 episodes), compared with 78.8% (from 6.5 to 1.5 episodes) with behavioral and drug therapy.3 The mean voiding frequencies were lower for behavioral therapy alone compared with drug therapy alone (P < .001).3
Neurotoxin Injections
A third-line therapy available for urge incontinence is neurotoxin intradetrusor muscle injections such as onabotulinumtoxinA (BTX-A).4 BTX-A injections reduces anticholinergic use by over 50% 3 months postinjection.4 The efficacy following the first injection persists across multiple treatment cycles.5 There was a 33% reduction in the number of voids per day for those who had >11 voids per day [80% of those participants decreased to 1 to 7 voids per day](P < .001), a 23% reduction in nocturia (P < .001), and the total dry rate increased from 20% to 60% (P = .04) at 3 months after BTX-A.4 Unfortunately many of the studies available do not compare with placebo group. Adverse effects are in Table 2.
Management Options for Urinary Incontinence
Neuromodulation
If OAB is unresponsive to first- and second-line therapies, neuromodulation techniques like percutaneous sacral nerve stimulation (PSNM) and percutaneous posterior tibial nerve stimulation (PTNS) can be used. PSNM involves a permanent implantable lead at S3 nerve transmitting electric stimulation to control the bladder and pelvic floor muscles.6 When comparing PSNM versus medications, up to 56% of participants achieved continence and 88% had improvement in OAB symptoms.6 PTNS implants an electrode above the medial malleolus activating the tibial nerve over twelve weekly 30-minute sessions.6 PTNS has comparable therapeutic effects with antimuscarinic agents on improvement of OAB symptoms with fewer incidence of adverse effects and less discontinuation than antimuscarinic agents.7 However, some studies show no difference in incontinence and urgency episodes between PTNS and placebo groups.6 Adverse events are in Table 2.
Vaginal Laser Therapy
A third-line option for OAB in women is vaginal laser therapy (common lasers include CO2 and Er:YAG lasers). However, it is unclear how promoting vaginal epithelium thickness and collagen remodeling improves OAB symptoms and lacks high-quality supporting evidence.8 About 60% of patients treated with Er:YAG vaginal laser therapy were satisfied and reported an improvement in OAB over 3 months, but results were not sustained at 12 months.8 A 2023 systematic review and meta-analysis on CO2 laser therapy, suggests that the therapy results in changes in questionnaires and distress scores; not much focus on improvement in number of voids.9
Acupuncture
For OAB, there is a lack of supportive evidence between true and sham acupuncture.10
Urge Incontinence and Overactive Bladder
Pharmacotherapy for OAB
When first-line options do not adequately address OAB symptoms, pharmacotherapy is the next step. First, identify patient medications which may contribute to OAB and modify where appropriate.
Antimuscarinics
Antimuscarinics reduce OAB symptoms by inhibiting involuntary bladder contractions and relaxing detrusor smooth muscle. Medications include oxybutynin, darifenacin, solifenacin, tolterodine, fesoterodine, and trospium. Systematic reviews have concluded there is similar efficacy among agents in reduction of symptoms.2 A recent Cochrane review found that anticholinergic drugs probably lead to a slight reduction in the number of micturitions per 24 hours compared with placebo (MD 0.85, 95% CI 0.98 to 0.73; P < .00001) and most likely lead to a slight reduction in urgency episodes per 24 hours at the end of treatment compared with placebo (MD 0.85, 95% CI 1.03 to 0.67; P < .00001).11 Medication selection should account for patient preference, adverse effect history, comorbidities, route of administration, and cost.
Side effects are noted in Table 2. Lack of tolerability is one of the most common reasons for discontinuation and persistence rates at 1 year range from 15 to 25%.12 Longer-acting once-daily formulations are less likely to cause severe adverse effects. Topical oxybutynin formulations have a lower incidence of dry mouth and constipation.13 Although efficacy across various formulations is similar, affinity for muscarinic receptors, and therefore side effect profiles, differ among agents. For patients experiencing an inadequate response or intolerable side effects, switching to a different agent may be beneficial.
Of note, antimuscarinics are on the Beers list for older adults with dementia, cognitive impairment, delirium, or high risk of delirium. The total anticholinergic load should be assessed when considering long-term use of antimuscarinics in this population.
β-3 Agonists
β-3-agonists relax the detrusor smooth muscle during the bladder fill-void cycle and increase bladder capacity to relieve OAB symptoms. The primary advantage of β-3 agonists is avoiding anticholinergic side effects. The two available agents are mirabegron and vibegron (new agent released in 2020) which may take up to 8 weeks for therapeutic impact. Comparative studies have shown β-3-agonists to be as efficacious as antimuscarinics in symptom reduction and have better 12 month adherence.14 In a trial examining 75% reduction of urge incontinence episodes at 12 weeks, the endpoint was achieved in 52% of the vibegron group (NNT = 7), 48% in the tolterodine group (NNT = 10), and 37% in the placebo group (P < .0001).15 A recent 2023 randomized control trial showed both mirabegron at 50 mg and vibegron at 50 mg improved OAB symptoms and the parameters of voiding diary equally in postmenopausal women with treatment naïve OAB.16 See side effects in Table 2 and NNT in Figure 2.
Treatment modalities and numbers needed to treat.
Combination Therapy
Combining an anticholinergic drug and a β-3 agonist may be considered when individual agents lack efficacy or if increased antimuscarinic doses are intolerable. Solifenacin 5 mg plus mirabegron 25 mg or 50 mg was significantly better than monotherapy in decreasing episodes of urinary incontinence, urgency and nocturia in a 24-hour period after 12 weeks of continuous therapy.17
Stress Urinary Incontinence
Mechanical Devices
Mechanical devices such as vaginal cones, pessaries, and urethral plugs may be effective in patients with symptomatic pelvic organ prolapse (POP) and those who may not be surgical candidates.18 However, poor long-term adherence at 2 years may be associated with women with SUI after reduction (1-hour pad test >10 g vs ≦10 g, 57.1% vs 84.3%, P = .027) or age under 60 (age ≦60-year-old vs >60-year-old, 58.3% vs 83.0%, P = .04).19 No adverse effects were noted.
Pelvic Floor Muscle Training
The recommended first-line treatment for women with SUI or mixed urinary incontinence is PFMT, composed of exercises to improve strength, endurance and/or relaxation.19 While there is not consensus about the most effective intervention for SUI due to the heterogeneity of studies and lack of large RCTs, PFMT programs generally include 6 to 20 repetitions of phasic contractions of 1 to 5 seconds, followed by relaxation time equal to or twice as long, performed 2 to 3 times weekly.20 After PFMT, the median 1-hour pad test decreased by 17.8 g (P < .001) and median social activity index (scale from 0 = cannot undertake any social activity to 10 = does not have any problem) increased from 4.5 to 7.5 (P < .001).20 No adverse effects were noted. A 2022 Cochrane systematic review reported PMFT is more effective than control at achieving cure and improving symptoms and quality-of-life measures in women with all types of urinary incontinence. PFMT for all types of urinary incontinence is more effective if it is more intense, done more frequently, and performed with individual supervision.21
Electric Muscle Stimulation
With electric muscle stimulation (EMS), electric impulses are applied directly to the striated pelvic floor muscle (PFM). While the mechanism of action is unknown, it is theorized that intravaginal EMS stimulates the efferent motor fibers of the pudendal nerve, facilitating PFM contraction, leading to muscle hypertrophy and increased urethral pressure.20 After PFMT with estriol therapy and EMS, postmenopausal women reported a 59% decrease in SUI symptoms compared with 10% decrease with estriol alone (P < .01).20 This intervention may be an effective adjunct therapy for women who cannot actively contract PFM. Electric stimulation is more beneficial than control at achieving cure or improving symptoms in patients with stress urinary incontinence and improving symptoms in women with urge urinary incontinence.21
Biofeedback
Another adjunct therapy to PFMT is BF, a training through isolated or associated contractions in which information about an unconscious physiologic process becomes clear through auditory or visual signals that can then be modified.20 There are two types of BF: the manometric which captures pressure and the surface electromyographic which captures electric activity.20 In the manometric type, a perineometer measures the ability of pelvic floor muscles to develop vaginal tightness. Because increases in vaginal pressure can be due to abdominal muscle contraction, the perineometer measures the muscle function of the pelvic floor.20 After BF training in combination with PFMT, the median 1-hour pad test decreased by 19.3 g (P < .001) and median social activity index increased from 3.5 to 8.1 (P < .001).20
Acupuncture
Available evidence supports acupuncture and electroacupuncture (EA) for SUI. Acupuncture reduced urine leakage in pad 2.67 (95% CI, 4.05 to 1.29) and the validated International Consultation on Incontinence Questionnaire – Short Form (ICIQ-SF) score 3.46 (95% CI, 3.69 to 3.22).22 EA involves electric stimulation through acupuncture needles. A meta-analysis of two moderate-grade-quality studies involving 553 women with SUI demonstrated that the EA group reported a ≥ 50% reduction in UI episodes than in the sham group during the 27 to 30-week follow-up period (RR 1.73 [95% CI 1.46 to 2.04]; P < .00001.23 When combined with PFMT, EA is beneficial for improving SUI in women compared with medications.23
Overflow Incontinence
Women
In women, bladder outlet obstruction is typically caused by fibroids or POP. In these cases, treating the underlying pathology with medication, surgical intervention or pessary devices can be curative.
Men
In men, obstruction is usually caused by benign prostatic hyperplasia (BPH).24 α-1-blockers relax the smooth muscle of the bladder neck and prostate to allow passage of urine. These medications should be used in patients with moderate to severe lower urinary tract symptoms (LUTS).24
When choosing an α-1-blocker for the treatment of LUTS/BPH, the AUA recommends reviewing the side effect profiles of options.25 Selective α-1a blockers tamsulosin and silodosin are less likely to cause orthostatic hypotension compared with nonselective α-1 blockers like terazosin and doxazosin.25 Selective α-1a blockers should be considered in patients on multiple hypertensive medications or those at risk of orthostatic hypotension. If a patient on α-1 blockers is bothered by ejaculatory dysfunction (EjD), switching to an α-1 blocker like alfuzosin is recommended.25
If a patient is still symptomatic with α-1-blocker therapy, a 5-α reductase inhibitor (5-ARI) like finasteride or dutasteride should be used in patients with a prostate volume of > 30 mL on imaging or a prostate specific antigen (PSA) > 1.5 ng/dL.25 Since 5-ARIs reduce PSA by 50% after 3 months of use, it is important for clinicians screening for prostate cancer to double the PSA in patients on chronic 5-ARI therapy for accurate assessment. Studies have shown a delay in prostate cancer diagnosis when physicians are unaware of this effect.25
In patients with LUTS/BPH and erectile dysfunction, daily tadalafil 5 mg should be offered to help with both conditions.25
Older Adults
In the elderly, often defined in some studies as people > 55 years old26, overflow incontinence can be attributed to a variety of medications that cause reduced detrusor activity and subsequent urinary retention.27 Patients with underlying BPH are at highest risk of drug-induced urinary retention leading to overflow incontinence. The most common medications to cause acute urinary retention are drugs with anticholinergic effects, including antihistamines, antipsychotics, tricyclic antidepressants, antiparkinsonian agents, and anticholinergics for COPD/asthma or OAB.27 Males over the age of 45 years on calcium channel blockers and anticholinergic medications have tripled the risk of urinary retention.27 Physicians should use these medications judiciously in patients with BPH or those at risk for urinary retention.
Neurogenic Bladder
Current guidelines recommend clean intermittent catheterization (CIC) over indwelling and suprapubic catheters for neurogenic lower urinary tract dysfunction (NLUTD).28 Catheterization should occur 4 to 6 times daily to relieve bladder pressure, avoid infection, and prevent upper urinary tract damage from hydronephrosis. Patients should be instructed to wash their hands and genitals with soap and water before catheter insertion.29 Sterile gloves and technique are not needed. If a prelubricated catheter is not used, patients should be instructed to apply water-based lubricant to the distal two inches of the catheter before insertion.29
A common complication of CIC, CAUTI. Hydrophilic prelubricated single-use catheters are less likely to cause CAUTI compared with standard polyvinyl chloride multi-use catheters.30 The rate of asymptomatic bacteriuria (35.2% vs 36.8%, P = .877) is similar in both groups.30 Since the presence of asymptomatic bacteria is common in CIC patients and does not require treatment, urine should only be tested for infection if the patient is symptomatic.
Conclusion
Urinary incontinence management varies depending on the type of incontinence and severity of symptoms. First-line treatment for OAB and SUI is nonpharmacologic management. Pharmacologic management with α-1-blockers is recommended as first-line treatment for moderate to severe overflow incontinence from BPH. Drug side effects can often lead to discontinuation due to poor tolerability. β-3 agonists tend to have less anticholinergic side effects compared with antimuscarinics for OAB. Third-line therapies are for those who fail conservative and pharmacologic therapies and lack high-grade evidence. Future advancements in incontinence management include platelet-rich plasma (PRP). A recently published 2024 randomized trial suggests that periurethral PRP injections had a good safety profile and were superior to sham injections in improving SUI symptoms.31 Clinicians should assess type of incontinence, patient goals, side effect profile, and tolerability to determine the most appropriate option.
Acknowledgments
We acknowledge medical librarian Robin Sewell for her assistance with the literature search.
Notes
This article was externally peer reviewed.
Funding: None.
Conflict of interest: Dr. Nguyen owns equity in Abbvie. The other authors have no conflicts of interest.
To see this article online, please go to: http://jabfm.org/content/37/5/909.full.
- Received for publication December 15, 2023.
- Revision received March 28, 2024.
- Accepted for publication April 1, 2024.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.
- 44.
- 45.