Abstract
Background: The 2022 Centers for Disease Control’s “Clinical Practice Guidelines for Prescribing Opioids for Pain in United States” called for attention and action toward reducing disparities in untreated and undertreated pain among Black and Latino patients. There is growing evidence for controlled substance safety committees (CSSC) to change prescribing culture, but few have been examined through the lens of health equity. We examined the impact of a primary care CSSC on opioid prescribing, including by patients’ race and sex.
Methods: We conducted a retrospective cohort study. Our primary outcome was a change in prescribed morphine milligram equivalents (MME) at baseline (2017) and follow-up (2021). We compared the differences in MME by race and sex. We also examined potential intersectional disparities. We used paired t test to compare changes in mean MME’s and logistic regression to determine associations between patient characteristics and MME changes.
Results: Our cohort included 93 patients. The mean opioid dose decreased from nearly 200 MME to 136.1 MME, P < .0001. Thirty percent of patients had their dose reduced to under 90 MME by follow-up. The reduction rates by race or sex alone were not statistically significant. There was evidence of intersectional disparities at baseline. Black women were prescribed 88.5 fewer MME’s at baseline compared with their White men counterparts, P = .04.
Discussion: Our findings add to the previously documented success of CSSCs in reducing opioid doses for chronic nonmalignant pain to safer levels. We highlight an opportunity for primary care based CSSCs to lead the efforts to identify and address chronic pain management inequities.
- Family Medicine
- Gender
- Health Equity
- Intersectionality
- Logistic Regression
- Opioids
- Pain Management
- Patient Care Team
- Physician's Practice Patterns
- Race
- Registries
- Retrospective Studies
- Substance Use Disorders
Introduction
In 2016, the Centers for Disease and Control and Prevention (CDC) released guidelines for safer opioid prescribing for patients with nonmalignant chronic pain1 with a goal to reduce the risks associated with chronic opioid therapy, including opioid use disorder, overdose, and death.1 The guidelines advised: a) prescribing opioids in the lowest dose possible, b) frequent clinical assessment of risks and benefits when opioid dosages exceed 50 morphine milligram equivalents (MME)/day, and c) avoid prescribing or carefully justify dosages over 90 MME/day.
Many practices adopted the guidelines and incorporated interdisciplinary committees to review patient care and provide recommendations to primary care clinicians (PCCs).2⇓–5 Although literature quantifying benefits and outcomes of such committees are spare, some studies demonstrate guideline acceptance by clinicians and overall lower prescribed opioid doses for patients. Adawallah et al found a significant decrease in the average opioid dose (–66.6 MMEs) in a cohort of primary care patients reviewed by a safe prescribing committee during a 4-year period, P<0.001.5
Despite emerging evidence on the efficacy of the committees to promote safer opioid dosing, the literature lacks findings through the lens of health equity and disparities. Research has documented sex and racial differences in chronic pain management; Black patients across multiple clinical scenarios, including emergency, surgical, and primary care settings, are treated and evaluated for pain less frequently, resulting from structural and/or individual racism.6⇓⇓–9 Implementing opioid safety committees may inadvertently exacerbate existing disparities in dosages and access. Our study aimed to determine the impact of a primary care based CSSC on opioid prescribing, including by patients' race and sex. We operationalized the race and sex variables as unmeasured proxies for exposure to racism and sexism.
Methods
Background
In 2017, our Family Medicine (FM) residency training practice in western New York initiated a CSSC, a multidisciplinary group to advise clinicians and increase concordance with the 2016 CDC guidelines prescribing opioids for nonmalignant chronic pain. The team included Family Medicine residents, nurse practitioners, and attending physicians in addition to behavioral health clinicians, a care coordinator, data analyst, and a clinical pharmacist. The CSSC met monthly to review and discuss patients with chronic pain managed with opioids. After discussion, the team provided a written summary of recommendations to the prescribing clinician to aid in aligning their treatment with new CDC guidelines.
In addition to the committee’s case review, the practice adopted the following clinical policies for patients prescribed an opioid, patients should: 1) receive, review and agree to a controlled medications agreement; 2) receive a prescription for naloxone and have a trusted person be trained in its use; and 3) agree to undergo periodic urine testing for controlled medications. Patients prescribed ≥ 90 daily MMEs were required to attend an evaluation with a credentialed alcoholism and substance abuse counselor (CASAC) to assess for substance use disorders. The practice developed a patient registry that provided each clinician with a summary of the patients on their panel and the total opioid dose prescribed and the adherence of the clinician to the above interventions. The associate medical director, who led the CSSC, provided feedback to clinicians with patients who had potentially unsafe opioid prescriptions.
Setting and Sample
We conducted a retrospective cohort study of patients listed on CSSC registry. The registry included all patients in the practice prescribed a controlled medication. Our study’s cohort included: adult (≥ 18 years) patients with nonmalignant chronic pain prescribed a controlled medication ≥ 90 MMEs for ≥ 90 days and included all patients with a visit in fiscal year 2017 (baseline) and at least 1 subsequent visit in 2021 (follow-up). We chose the 4-year period to account for the time needed to safely taper controlled substances among patient who entered the cohort at different times. Controlled medications included opioids, stimulants, sedative-hypnotics, and pregabalin. For this analysis, we focused on opioid analgesics, measured in Morphine Milligram Equivalents (MME) to allow standardized comparison.
In 2017, our practice initiated a CSSC. The goal of the CSSC was to align with the 2016 CDC guidelines related to prescribing opioids for nonmalignant chronic pain. It was composed of a multidisciplinary group of medical and behavioral health clinicians, care coordinators and medical residents. They convened monthly for 50 minutes to discuss specific patient cases that involved potentially unsafe opioid prescriptions (≥ 90 MMEs). After discussion, the CSSC provided a written summary of their recommendations to the prescribing clinician.
Measures
The practice’s data coordinator abstracted anonymized CSSC data from the electronic medical record (EMR).
Patient Characteristics
Patient characteristics available from the electronic health record included age, sex at birth (male, female) and race (Black, White and unknown) derived from the electronic health record, which are presumably but not necessarily self-reported.
Dose Reduction
Our primary outcome measure was a change in MME dose from baseline to follow-up; we converted all doses of opioids to standard MME’s using a standard conversion factor10.
Dosing Disparities
We stratified our sample into 4 subgroups by sex (male vs female) and race (White vs non-White) to examine potential evidence of disparities in dose changes. We compared the differences in dose changes between the subgroups at baseline and follow-up.
We also examined potential intersectional disparities. An intersectional lens recognizes that social categories are mutual and the experience of 1 social category (eg, race) may differ across other categories (eg, sex).11 Therefore, we described the joint disparity as the difference in MMEs at baseline and follow-up between those dually marginalized (Black women) to those that do not belong to either marginalized group (White males).12
Analyses
We performed descriptive and bivariate analyses to determine differences in dosing at baseline and follow-up. We summarized the data using frequencies and proportions, and numeric measurements with means and standard deviations. We used paired t test to compare changes in mean dose from baseline to follow-up among subgroups. We used logistic regression to determine if patient characteristics were associated with being prescribed a dose of < 90 MME at follow-up. P values of <0.05 were considered statistically significant. We performed all analyses using Stata 12.0 (College Station, TX).
The University of Rochester’s Institutional Review Board approved the study.
Results
There were 93 patients in our cohort. Table 1 shows the demographics of the cohort. Most patients were White, female and had 6 visits with a primary care clinician during the study period.
Cohort Characteristics 2017–2021
The mean opioid dose in the cohort decreased from nearly 200 MME at baseline to 136.1 MME at follow-up, Table 2. Thirty percent of patients had had opioid dosing reduced to under 90 MME by follow-up. Ninety-five percent had a dose change. Seventy-eight patients (84%) had a decrease in dose, (n = 7, 8%) had an increase and (n = 8, 9%) had no change. The mean decrease and increase in MMEs were −82.9 and 83.7, respectively. Patient demographics were not statistically significant predictors of being prescribed <90 MME at follow-up.
Changes in MMEs from 2017–2021
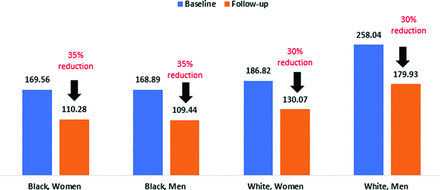
At baseline, White men were prescribed higher doses than other groups at baseline (258 MME) and experienced the greatest absolute dose reduction, a mean reduction of 78.1 MME, Figure 1 Black men and women had a 5% higher rate of reduction compared with White men and women, but the reduction rates by race or sex were not statistically significant.
This graph shows the changes in morphine milligram equivalents (MME) dose in 2017 and 2021 by sex and race. All groups had dose reductions ranging from 30% to 35%. The greatest reductions were among Black men and women.
Table 3 depicts the comparison in mean dose by race and sex subgroups at baseline and follow-up. At baseline, Black women were prescribed on average 88.5 MME’s fewer compared with their White men counterparts; this number represents the joint disparity based on race and sex, P = .04. At the follow up period, the joint disparity was reduced to 69.65 at follow-up and was no longer statistically significant.
Single Group and Intersectional Disparities
Discussion
Our findings add to the previously documented success of CSSCs in reducing opioid doses for chronic nonmalignant pain to safer levels.13,14 A 30% reduction in dose has been reported as clinically significant.15 The overall mean opioid dose in this study was reduced by 28%, to 136 MMEs. At baseline, White men were prescribed the highest mean dose whereas Black women were prescribed the lowest mean dose. The joint disparity for Black women was statistically significant at baseline, but not at follow-up. Absolute dose reductions were highest for White men and relative dose reductions were greater for Black patients in general. However, these findings were not statistically significant. Previous studies have shown that White patients were more likely to receive high dose opioids for pain than patients who are Black, Latinx, or Asian.16⇓–18 Women receive lower MME for noncancer pain and self-administer fewer MME based on patient controlled analgesia devices.19 The extent to which these differences reflect implicit gender bias, gender roles, differences in opioid receptors or hormonal differences remains is not clear.20⇓–22
We did not establish our CSSC with a health equity focus. Our registry demonstrated higher prescribed doses for White patients, consistent with other studies. Buonora et al found that Black, female patients ≥ 60 years old, were more likely to experience dose reductions over a 2-year period.15 In 2022, the CDC updated their Clinical Practice Guideline for Prescribing Opioids for Pain in United States. The updated guidelines no longer recommend upper limits for dosages but suggest cautious tapering of high dose opioid medications and provide strategies on how to appropriately discuss risks and benefits of opioid use with patients. The new guidelines acknowledge the potential harms of dose reductions including “untreated and undertreated pain, serious withdrawal symptoms, worsening pain outcomes, psychological distress, overdose, and suicidal ideation and behavior.”23 These updated guidelines also call out the need for attention and action toward reducing disparities in untreated and undertreated pain among Black and Latinx patients. To our knowledge, our work is among the first to examine the effect of broad implementation the CDCs guidelines at the intersection of marginalized groups in primary care.23
The strengths of the study include real-world longitudinal data on patients receiving chronic opioid pain treatment during a period when the CSSC was operational. Limitations include small samples of subgroups and limited categories for race, ethnicity, and gender and their intersectionality. We were unable to assess clinical appropriateness given insufficient data in the registry. We cannot exclude the possibility of confounding factors that might account for differences in baseline doses or dose reductions.
We did not observe statistically significant differences in relative dose reductions by race. However, our modest subgroup sample sizes may have precluded our detection of such a difference. Most importantly, we did not assess any patient-reported outcomes or patient-reported experience of care. Data suggest caution in tapering. In a retrospective cohort study, discontinuation of chronic opioid therapy for pain did not reduce risk of death, but rather was associated with increased risk of overdose death.24 In a secondary analysis of a large Veterans Administration trial, the expansion of mandated case review of high-risk patients prescribed opioids for pain resulted in an increase in all-cause mortality among patients recently diagnosed with opioid use disorder.25 Given the challenges of identifying opioid use disorder among patients receiving opioids for chronic pain, cautious slow reduction dose reduction when indicated is prudent.26,27
In this study, Black patients had lower doses to start and slightly higher rates of reduction. Our findings highlight the importance of addressing health equity early when implementing CSSCs. The inequities in baseline dosing rates require strategies for tapering chronic pain medications to be more nuanced and equity focused. General implementation of guidelines without special attention to certain patient populations can have unintended consequences and exacerbate disparities in pain control over time. Differences in initial dosing highlight an opportunity for primary care to lead the efforts of equitable CSSCs. We encourage future studies to examine our findings in the context of a larger sample size.
Notes
This article was externally peer reviewed.
Conflict of interest: None.
Funding: Support for this project was provided by the McDaniel-Farley Psychosocial Medicine Faculty Development Award.
To see this article online, please go to: http://jabfm.org/content/37/3/383.full.
- Received for publication June 7, 2023.
- Revision received November 3, 2023.
- Revision received November 27, 2023.
- Accepted for publication December 11, 2023.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.