Abstract
Family physicians take pride in addressing the totality of a patient’s experience of disease and are skilled in a multidisciplinary approach to care. As such, they have an important role to play in managing adult cancer pain. Although 75% to 90% of cancer patients could receive adequate pain relief from routine pharmacologic therapies delivered by family physicians, pain continues to be undertreated in this population. Pain is a global experience affecting the whole person. Our role as patient advocates and educators makes us well suited to participate in the current national attempt to redress the lack of attention to this important component of suffering. This article reviews commonly seen cancer pain syndromes, with specific recommendations concerning assessment, reassessment, management, and indications for consultation.
Family physicians aspire to care for people from birth to death, recognize the importance of continuity of care, and take pride in addressing the totality of a patient’s experience of disease. As such, pain management is an important component of routine primary care, including the care of patients with cancer, even as the cancer progresses. Comprehensive cancer care requires a multidisciplinary team that may include nurses, social workers, pharmacists, and chaplains, among others. Family physicians are trained and experienced in this approach to health care.
Rather than provide an exhaustive description of cancer pain, this article is designed to guide treatment decisions for commonly seen syndromes and alert the family physician to the conditions that warrant specialty consultation. It considers management from the viewpoints of severity, quality, and cause of cancer pain.
Prevalence
Epidemiologic data that estimate the prevalence of cancer pain are difficult to interpret because of variations in methods, setting, tumor type, and patient populations. Nonetheless, it is reasonable to estimate that chronic pain occurs in approximately 30% to 50% of patients receiving active treatment for a solid tumor and 60% to 90% of cancer patients with advanced disease.1 In those cancer patients, 75% to 90% could receive adequate pain relief from routine, pharmacologic therapies2 delivered by primary care providers.3 Despite this, pain continues to be undertreated in this population. One study4 found that minority status, female sex, and history of substance abuse were among the factors associated with undertreatment of pain.
Definitions
Effective management of cancer pain depends on multiple skills and involves clinical judgment at each juncture. A closer look at the definition of pain illustrates many of the issues involved: “Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.”5 In other words, pain goes beyond tissue damage to involve the whole person. As Galer and Dworkin6 point out, because pain is “unpleasant,” it usually provokes a behavioral response, and because it is “emotional,” it is interpreted according to the meaning the person attributes to it.
The issue of meaning plays a particularly important role in patients suffering pain in the context of a potentially life-threatening disease. As cancer progresses, many patients become increasingly disabled. Their roles in the family may be significantly altered, and loss of employment or routine duties may erode a person’s sense of identity, self-worth, and self-esteem. All these shifts affect the meaning of the experience. In the context of care for the terminally ill, the biopsychosocial model has to be expanded to include the person’s existential, spiritual, and community concerns. This might be called the domain of the transcendent, meaning everything with which the person identifies or to which the person feels connected, beyond the self. Pain management is unlikely to be successful without specifically addressing this domain, but there is little or no research about how best to address it. Some patients and physicians can explore these issues together. Other patients are more comfortable speaking to chaplains or social workers. The main point here is that, in line with the philosophy of Family Medicine, we need to find ways to attend to the larger context in which the patient’s experience is located.
The goals of cancer pain management are to prolong survival, maximize comfort, and optimize function. The World Health Organization’s systematic approach to pharmacologic management of cancer pain7 was endorsed by the United States Agency for Health Care Policy and Research, and has influenced or been the model for all subsequent systematic approaches to drug therapy for pain. However, the goals of cancer pain management require going beyond drug therapy. For some, this has meant adding interventional techniques. For family physicians, ongoing attention to advance care planning, education, and support are equally important and are considered, in themselves, effective modalities for pain relief.
Evaluation
The three most important aspects of pain management are assessment, management, and monitoring.8 Whenever possible, the underlying cause of pain should be identified and treated. However, it is cruel and pointless to delay the treatment of pain as a symptom until a definitive diagnosis is established. Diagnosis and treatment should be initiated simultaneously.
Most cancer pain is directly related to the neoplasm, but other causes include cancer treatment, diagnostic procedures, progressive debilitation, and chronic premorbid conditions. Tumor growth leads to pain by obstructing, deforming, compressing, or invading somatic, visceral, or neural structures. Guidelines for assessing and monitoring pain by severity have already been given in the introductory article of this supplement. Below is a brief review of the more common and important cancer pain syndromes and their presentations, with attention to the red flags that indicate the need for consultation with other specialists.
Management
Overview
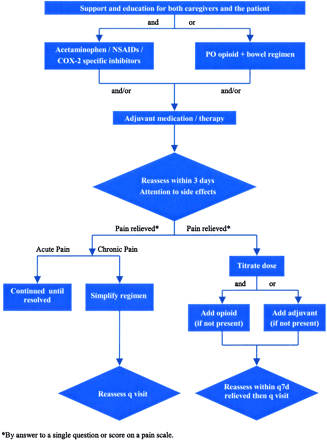
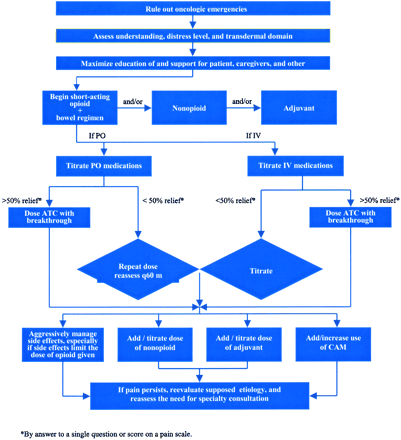
Asking about pain on every visit underscores the physician’s commitment to patient comfort. Figure 1 presents an algorithm for treating mild cancer pain. In cancer patients, even mild pain may trigger fears of death, disability, or progression of disease. Creating the opportunity for patients to discuss the meaning of their pain is an important part of its treatment. Nonopioids generally resolve the physical symptoms of mild pain. As in every other aspect of care, however, respecting the patient’s preferences improves adherence and possibly effectiveness. For patients who are reluctant to take medications, complementary and alternative therapies may be suggested. Those who take comfort in words may find counseling helpful. Those for whom physical activity is highly valued may find that yoga or physical therapy provides relief. Figure 2 presents an algorithm for managing moderate to severe cancer pain. The first step is to rule out oncologic emergencies. When pain is moderate to severe, opioids are almost always part of the treatment plan. The doses required to control cancer pain may be much higher than those used for nonmalignant pain. For example, several grams of morphine sulfate per day may be required to achieve acceptable analgesia.
Mild cancer pain treatment algorithm.
Moderate and severe cancer pain treatment algorithm.
At high doses the side effects of opioids become increasingly troublesome. Patients often do not report side effects unless specifically asked. Routine inquiry about sedation, constipation, nausea, vomiting, and pruritus ensures attention to whether the patient is suffering from other disturbing symptoms in order to maintain analgesia. There are 5 main strategies for handling side effects.9 First, one can maintain the same analgesic agent but change the dose or dosing frequency. Sometimes, a 25% decrease in opioid dose maintains analgesic efficacy but makes side effects manageable. If the decreased dose provides insufficient analgesia, the agent may be given more frequently. On the other hand, short-acting formulas produce peaks in serum levels, which may be the source of the side effect. Changing to long-acting formulas or to a continuous intravenous or subcutaneous infusion, which promote a more constant serum level, may eliminate the side effect. The second strategy is to rotate to another opioid. Persons vary in their responses to individual drugs; often, changing to another agent within the same class is sufficient to provide relief. If pruritis and urticaria are disturbing, it should be noted that fentanyl has a low potential to release histamine. A third method is to change the route of administration. Subcutaneous, intravenous, and transdermal routes may cause fewer gastrointestinal symptoms than the oral route. Intraspinal administration of opioids allows a dramatic dose reduction, with consequently lower drug levels in the brainstem. This often eliminates sedation, nausea, and vomiting.
The fourth strategy for managing opioid side effects is to add nonopioid analgesics or coanalgesics and nondrug methods of pain control to reduce the total dose of opioid given. Acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and caffeine, for example, act synergistically with opioids and may provide sufficient relief to permit decreased doses of opioids. Caffeine belongs to a category commonly called “coanalgesics.” These are agents that act to enhance analgesia, are themselves analgesic, at least partially, or counteract the side effects of analgesics. Other coanalgesics include tricyclic antidepressants, antiepileptics, and glucocorticoids. Because physical, psychological, and/or complementary modalities may also be opioid sparing, they may be said to act as coanalgesics. Finally, it may be necessary to add an agent to specifically counteract a side effect of the opioid. See Table 1 for a partial list of agents commonly used for selected side effects of opioids.
Selected Agents for Managing Side Effects of Opioids
Pain Syndromes Whose Etiology Is Tumor-Related
As a rule, acute pain in a patient with a history of cancer should be considered a recurrence or progression of disease until determined otherwise, and it should prompt immediate diagnostic studies. Family physicians should be able to recognize and respond to the most common oncologic emergencies, including brain metastases, spinal cord compression fracture, high risk of fracture of a weight-bearing bone, and obstruction of a hollow viscus (Table 2).
Oncologic Emergencies
Somatic Pain Syndromes
Bone Pain
Bone pain syndromes are probably the most prevalent somatic pain syndromes in cancer patients. Stable bone pain without progressive neurologic change can usually be managed medically. However, if stable bone pain is not responding to treatment, imaging is warranted. Local pain and referred pain produced by metastases generally will be managed by radiation [SORT B].10 Plain roentgenograms, the initial step in diagnosis, are more specific than sensitive; they do not detect small metastases. Scintigraphy (radioisotope scanning) is more sensitive but is difficult to interpret when used for regions of the body that have previously been irradiated, and a positive study may be the result of numerous other causes. For these reasons, computed tomography or magnetic resonance imaging may be more useful if plain films are negative and clinical suspicion warrants further imaging [SORT B].11
External beam radiation is usually the treatment of choice for focal, painful skeletal metastases [SORT B].12–14 Almost half of patients will get complete relief of pain and as many as 90% will attain partial relief.15 NSAIDs and COX-2-specific inhibitors are helpful in this situation because they inhibit the prostaglandin E2 produced by bone metastases.16 However, the risk of gastrointestinal bleeding is markedly increased when NSAIDS are used in combination with steroids, and it is reasonable to assume that there is increased risk with COX-2s.17 Opioids are generally effective for constant, aching bone pain; however, chronic, stable doses will not reduce pain on movement. For many people, pain on movement can be managed by using patient-controlled analgesia. By using an intravenous or subcutaneous route, an extra dose of opioid can be delivered on demand. The rapid onset achieved by these routes of administration is an advantage and, when pain is predictable, patients can premedicate before undertaking a painful activity.
Other treatment options exist for specific situations. When radiation has already been maximized, or metastases are too widespread for local treatment to be effective, radionuclides may be used to treat bone pain. Strontium-89 has been used as both an adjuvant to local radiation and a treatment for diffuse metastatic bone pain.18 As with radiation, it may take 2 to 3 weeks for the full effect to be felt. During this time, analgesics should be continued. Hormonal therapy is often effective for cancer patients with painful bony metastases. Bisphosphonates such as pamidronate disodium and zoledronate inhibit osteoclastic bone resorption. They are given intravenously and have been used successfully, most commonly for breast cancer, prostate cancer, and multiple myeloma patients [SORT A].19–23 The use of hormonal treatments and bisphosphonates is best managed in consultation with a medical oncologist.
Bone Pain Emergencies
Pathologic fractures present as pain with movement and are associated with local tenderness on palpation. An unstable fracture, particularly in a weight-bearing bone, may require surgical stabilization. Plain roentgenograms should be obtained immediately. The appropriate intervention depends on the clinical context. For a high-functioning person reasonably likely to survive the intervention, surgical stabilization is an ideal choice. In advanced disease, external immobilization may be a more reasonable approach, especially if the pain can then be adequately managed medically.
Epidural spinal cord compression (SCC) presents as progressive, central back pain radiating bilaterally. The pain is generally increased with recumbency or increased intra-abdominal pressure or is accompanied by bilateral sensory (and sometimes motor) changes in the lower extremities. SCC is a medical emergency; every patient with a history of cancer who presents with new or changing back pain having the above features should be evaluated for SCC [SORT B].24 The diagnosis should be confirmed with immediate imaging studies of the entire spine (magnetic resonance imaging) and consultation with radiation oncology, neurology, and oncology. If confirmed, there is good evidence for a loading dose of 96 mg of dexamethasone, then 96 mg per day (divided into 4 doses) for 3 days, tapered off over 10 days, but there is a high risk of serious complications [SORT B].25–29 There is lower risk but less effectiveness data for a regimen of 16 mg/day (divided into 4 doses), tapered off over 14 days [SORT C].30–32
Headache and Facial Pain Syndromes
New headache, increased frequency of headache, or headache qualitatively different from the past may signal brain metastases and should trigger automatic magnetic resonance imaging with contrast. Associated signs and symptoms include nausea, vomiting, lethargy, photophobia, and personality or mental status changes. Progressive neurologic findings with headache are medical emergencies.
Metastases to the base of the skull result in a number of specific syndromes. They present with facial pain or headache and a variety of neurologic findings related to the specific cranial nerves affected by tumor. These syndromes are difficult to treat and require consultation with pain specialists and surgeons to preserve as much function as possible.
Visceral Pain Syndromes
Visceral pain syndromes arise from acute obstruction of a hollow viscus or deformation of a solid viscus. The location determines the presenting signs and symptoms.
Emergent Visceral Pain Syndromes
Obstruction of a hollow viscus may be an emergency if it leads to perforation, ischemic necrosis, or organ failure. Intestinal obstruction presents as continuous or colicky pain and may be associated with nausea, vomiting, constipation, diarrhea, or distention. Onset may be sudden but is often insidious, developing over weeks to months. Because severe constipation with fecal impaction can mimic obstruction, a digital rectal examination is almost always part of the assessment. Surgery should be considered whenever a cancer patient develops obstruction for the first time. For patients with recurrent obstruction and advanced disease, medical management of symptoms is preferable [SORT B].33 Colicky pain responds to antispasmodic agents. Opioids relieve constant pain but also decrease peristalsis, which contributes to constipation. Antiemetics can usually control vomiting.
Nonemergent Visceral Pain Syndromes
Stretching of the liver capsule often produces a local, continuous, dull pain. Opioids are the first line of pharmacologic treatment for this type of pain [SORT B]. Steroids or nonsteroidal anti-inflammatory medications may reduce peritumoral edema and are an option for adjuvant treatment.
Neuropathic Pain Syndromes
Mononeuropathy, polyneuropathy, and radiculopathy generally respond well to medications. However, neuropathic pain is often difficult to control with opioids alone. In clinical practice, antiepileptic drugs have become the drugs of choice for lancinating pain and incident pain and are widely used for all types of neuropathic pain [SORT B].34–36 Gabapentin has an established track record and a good safety profile.37 Up to 6000 mg/day may be required, although approximately 3600 mg/day is generally sufficient for cancer pain. Numerous other antiepileptic drugs are also used.
Tricyclic antidepressants (TCAs) are still first-line agents to treat neuropathic pain from cancer [SORT B].38,39 They are inexpensive, can be given once daily, and their analgesic effects are often evident at doses much lower than those required for an antidepressant effect.40 The choice of agent depends on its side effect profile, especially degree of sedation, orthostatic hypotension, weight gain, and anticholinergic action. Amitriptyline produces the greatest degree of all these effects. Nortriptyline and desipramine are minimally sedating and anticholinergic, with only a modest effect on orthostatic hypotension and weight gain. Trazodone is as sedating as amitriptyline without anticholinergic activity and with minimal effect on orthostasis and weight gain.41 Because they have class 1A (sodium channel blocking) antiarrhythmic actions, tricyclics should be used with caution in patients with known arrhythmias or ischemic heart disease [SORT B].42,43 Increasing doses lead to increased side effects. If side effects limit the dose of TCAs, or depression is recalcitrant to treatment, clinicians often add a selective serotonin-reuptake inhibitor [SORT C].44,45 Because citalopram and escitalopram do not interfere with the metabolism of TCAs, they are the preferred agents to use with TCAs.
Plexopathies (involving major peripheral neural plexuses) and pain on movement are far more difficult to control.46 Severe neuropathic syndromes respond partially to opioids, but many cancer patients will not have neuropathic pain controlled using the WHO approach, especially in advanced stages of disease. This has led some clinicians to call interventional techniques the “fourth step” in the analgesic ladder.47
Interventional techniques are the province of anesthesiologists and interventional radiologists. Some surgeons and rehabilitation specialists are also skilled in these techniques. They include nerve blocks, spinal administration of anesthetics and other medications, and surgical procedures. These techniques are used when systemic medications fail to control pain adequately and when adequate pain control requires dosing systemic medications at levels that may produce unacceptable side effects.
A celiac plexus block is probably the most useful nerve block for tumor-related pain [SORT A]. The celiac plexus innervates the upper abdominal organs, so celiac plexus block is indicated for pain from pancreatic and other upper abdominal cancers.48 It is effective in 80% to 90% of cases, producing analgesia for 2 to 6 months. For patients with a life expectancy within this range, it should be considered a first-line treatment. Other plexus blocks are commonly done, but none are generally considered first-line treatment.
Intraspinal infusions allow reductions in oral and transdermal medications, with consequent reductions in side effects. Consultation with anesthesiologists is required. Neurosurgical and neuroablative techniques have the highest morbidity and mortality and are reserved for times when all else fails.
Conclusion
Family physicians can play an important role in managing adult cancer pain. Skills essential to Family Medicine, such as treating the patient within the context of family and community and using a multidisciplinary approach to chronic disease, are directly transferable to problem solving around cancer pain. Taking the time to learn or review the basic principles of pharmaceutical pain management, family physicians can advocate for and help provide their patients a more peaceful death.
Acknowledgments
I thank Peter Selwyn, Sean O’Mahony, and the members of the Department of Social and Family Medicine Writers’ Group for their comments and suggestions on earlier drafts of the manuscript.
Notes
The Family Practice Pain Education Project (FP-PEP) acknowledges an unrestricted educational grant from Pfizer to Cardinal Health to produce educational materials for primary care doctors about pain management. The information provided here is the opinions and research of the family physicians who served on FP-PEP.
This work was presented in part at the 2003 American Academy of Family Physicians (AAFP) Scientific Symposium.