Abstract
Background: Cigarette smoking rates remain disproportionately high among low income populations with unmet social and behavioral health needs. To address this problem, we sought to develop and evaluate the feasibility, acceptability, and preliminary effectiveness of a novel smoking cessation program for community health centers that serve these populations.
Methods: We implemented a randomized pilot trial of two smoking cessation programs in three county operated community health center (CHC) sites: (1) a systematic assessment of smoking habits and standard tools to assist with smoking cessation counseling (“Enhanced Standard Program” or ESP), and (2) another that added a structured assessment of social and behavioral barriers to smoking cessation, (“Connection to Health for Smokers” or CTHS). Clinical outcomes were evaluated between 10 to 16 weeks, supplemented with interviews of patient participants and health care team members.
Results: 141 adults were randomized and 123 completed the intervention (61 in ESP, 62 in CTHS). At follow-up, over half of participants reported ≥1 quit attempts (59.7% ESP and 56.5% CTHS; adjusted p = .66) while more in ESP (24.6% vs. 12.9%) were documented as not smoking in the last 7 days (adjusted p = 0.03). In addition to being in ESP, predictors of smoking cessation included higher baseline confidence in ability to quit (p = 0.02) and more quit attempts during the study (p = 0.04). Health care teams, however, generally preferred the more comprehensive approach of CTHS.
Conclusion: Lessons learned from this pilot study may inform the development of effective smoking cessation programs for CHCs that combine elements of both interventions.
- Community Health Centers
- Community-Based Participatory Research
- Counseling
- Health Disparities
- Practice-based Research
- Primary Health Care
- Smoking Cessation
- Social Determinants of Health
Introduction
Smoking prevalence among adults living in poverty remains disproportionately high.1 Community health centers (CHCs) are the primary source of health care for this population, and they can play a pivotal role in addressing this disparity.2 Yet, CHCs often lack resources and staff to deliver robust smoking cessation programs. Even where such programs exist, these programs provide little systematic attention to social and behavioral barriers to smoking cessation. As supported by growing evidence, optimal health care delivery for medically vulnerable populations should include a comprehensive approach that delivers guideline-based recommendations in the context of patient-reported social needs (e.g., housing security, food security, financial security, transportation) and behavioral health challenges (e.g., level of social isolation, exposure to violence, co-occurring substance use, or mental health conditions).3 Many of these social and behavioral conditions are known to interfere with planning for successful quit attempts and maintenance of smoking abstinence.4⇓⇓⇓⇓⇓⇓–11 To respond to this growing body of research, and at the request of our community stakeholders, we sought to implement and test two smoking cessation programs specifically designed for implementation by CHC health educators. One program was designed to assess smoking in the context of social and behavioral needs with computer-assisted priority setting and action planning (“Connection to Health for Smokers” or CTHS). The other program provided traditional patient education about smoking cessation without formal evaluation of contextual needs (“Enhanced Standard Program” or ESP). Our goal was to understand the relative feasibility, acceptability and efficacy of these two approaches in helping patients to succeed in setting and achieving short-term smoking cessation goals.
Methods
Setting and Design
This study was a partnership between the University of California San Francisco (UCSF) research team and Contra Costa Health Services (CCHS), a large county health system in Northern California. CCHS previously collaborated with the research team on the implementation and evaluation of a novel diabetes self-management program, which clinic leaders felt could be adapted to support smoking cessation activities.12 CCHS uses an electronic health record that provides point of care smoking assessment reminders and a registry of patients who smoke. CCHS selected 3 clinical sites to participate based on the availability of health educators at each site who could participate in the design and implementation of this research. Through an iterative process of engagement with CCHS health educators and clinic managers, we developed a 2-arm patient-level randomized trial to understand the relative feasibility, acceptability, and efficacy of “Connection to Health for Smokers Program” (CTHS) and “Enhanced Standard Program” (ESP) for assisting individuals in quitting or cutting down on smoking. Intervention outcomes were assessed 10 to 16 weeks postenrollment via participant self-report and review of electronic health records, plus exhaled carbon monoxide testing on a subsample of patient participants to corroborate reported smoking status at follow up. We also conducted postintervention qualitative interviews with a subsample of patient participants, clinic staff who worked directly with the patients, and clinic leaders who approved site participation in the study.
Interventions
Connection to Health for Smokers (CTHS)
CTHS included 5 core components: 1) an electronic patient health survey of smoking behaviors and social and behavioral needs, 2) a summary report for the patient and health educator to use as a tool for identifying needs and concerns, 3) an interactive goal-setting process that takes place between the patient and the health educator, which may include smoking cessation, cutting down on smoking, or working on other needs before trying to quit smoking, 4) an interactive motivational action planning process to address and support the goal selected, with structured computerized guidance for the patient and health educator including consideration of social and behavioral contextual needs and a printed action plan with arrangements for smoking cessation medication as needed, and 5) postvisit automated text messages linked to the action plan, with documented telephone or in person counseling from the health educator at least twice over a 10-week period following the intervention. A description of CTHS has been published elsewhere.13
Enhanced Standard Program (ESP)
We worked with our clinical partners to gather existing smoking cessation resources already used in their health system and created ESP, assembling them in a way that would have structural similarities to CTHS. ESP thus included the following 5 components: 1) an electronic patient health survey restricted to assessments of smoking behaviors, 2) a summary report for the patient and health educator to use as a tool to set goals for smoking cessation, 3) an educational video about the risks of smoking and the challenges of smoking cessation, 4) a booklet for the health educator to review with the patient and for the patient to take home and arrangements for smoking cessation medication as needed, and 5) telephone and in person follow up as needed.
Clinic Training and Patient Recruitment
Three primary care sites from CCHS delivered the study interventions, with between 3 to 6 health educators at each site participating in 4 hours of training. The training included information on tobacco cessation counseling best practices, the mechanics of using CTHS and ESP, and research study protocols led by the study investigators with expertise in primary care and smoking cessation (MP, JT). In addition, health educators in both arms were trained on offering nicotine replacement as an option to support cessation and facilitated prescriptions from the primary care team when requested. Health educators identified patients for outreach based on their existing clinic smoking registries and referrals from their primary care providers. Patients agreeing to meet with a health educator to discuss the possibility of smoking cessation were randomly assigned to either CTHS or ESP at the time of their visit. Randomization was stratified by primary care site using a computer-generated random number protocol in alternate blocks of 4. Participants were eligible for inclusion in the study if they were current smokers (smoked in the past 7 days days), aged 18 years or older, and able to speak and read in English or Spanish. Exclusion criteria included clinically diagnosed severe cognitive impairment or untreated psychosis. Health educators were observed completing the intervention with their first several participants, and they were instructed on how to put results into the medical record for viewing by each participant’s primary care provider. The intervention took approximately 30 minutes per patient in each arm. The research protocol was approved by the UCSF and CCHS institutional review boards and registered with clinicaltrials.gov.
Study Measures
Patient Reported Data
As part of the baseline assessment, participants in both intervention arms self-reported their: age, gender, ethnicity, height, weight, smoking history, number of days in the past month smoked, number of cigarettes per day on days smoked, time to first cigarette, other tobacco products used, history of quit attempts, plan to quit in the next 30 days (yes/no), plan to quit in the next 6 months (yes/no), and confidence in being smoke free in the next 6 months (0 to 10 scale).14 We further categorized a plan to quit in the next 30 days as “preparation,” plan to quit in the next 6 months as “contemplation” and no plan to quit as “precontemplation,” according to the Prochaska stages of change model.15,16 As part of the CTHS program only, participants additionally reported on aspects of behavioral and social risks including: (1) depression symptoms as assessed by the Patient Health Questionnaire (PHQ2, range 0 to 6, and if PHQ2 ≥ 3 followed by PHQ-8 excluding the question about suicidality, range 0 to 24);17,18 perceived general health (poor/fair vs good/very good/excellent); alcohol binge drinking more than 4 drinks in a day more than once a month (yes/no) and illegal drug use or use of narcotic prescription medication for nonmedical purposes in last year (yes/no); general life stress in the last week (yes/no); social isolation (talk to someone you feel close to ≤2 times/week); and current experience of social risks (yes/no) in each of the following areas: food insecurity, housing instability, limited access to health care due to transportation or cost, feeling unsafe at home, feeling unsafe in the community, or having utilities disconnected in the last year. CTHS smoking action plan data were electronically captured in a structured format to record: type of action plan, specific actions and goals, as well as smoking triggers and strategies to avoid, cope with, or escape from these triggers.19 Between 10 and 16 weeks after meeting with the health educator, patient participants in both arms were contacted by the clinic staff and research team and invited to repeat the smoking cessation questions, with additional questions to establish number, type, and duration of quit attempts, as well as the amount of time since the last cigarette at the time of follow-up. Change in weekly number of cigarettes was calculated between baseline and follow-up was calculated to report: (1) Any evidence of cutting back (≤ 1 or more cigarettes), (2) the percentage of cigarettes cut back, and (3) ≥50% cutting back (yes/no). Carbon monoxide breath testing was performed on a subsample of patients to confirm self-reported smoking status at follow-up.
Electronic Health Record
The electronic health record (EHR) for participants was reviewed by the research coordinator for the active intervention period (baseline visit to 16-week follow-up) and key data elements were extracted and coded in a structured dataset in REDCap software (projectredcap.org). Abstracted chart measures included participant tobacco outcome measures that mirrored the survey data to further augment understanding of smoking quit attempts, evidence of cutting back, and smoking status at follow-up (≥10 weeks post enrollment). In addition, the process measures of number of patient ambulatory care medical appointments during the intervention follow-up period (yes/no ≥2 appointments during the 0 to 10 week period in accordance with the study procedure) and prescription of any tobacco cessation products (yes/no) were recorded.
Qualitative Interviews
To assess intervention feasibility and acceptability we conducted brief (20 to 40 minute) qualitative interviews. We interviewed clinic administrators at each site who were directly responsible for supervising the health educators (n = 5). We interviewed health educators at each site with experience administering each program to at least 5 patients (n = 5). We interviewed patient participants individuals in both arms until reaching saturation (n = 31). Patient participants were asked a series of questions focusing on their overall motivation for participating in the study, their experience engaging with the program, and satisfaction with level of support they were offered to achieve their goals. They were also asked to identify potential program improvements. CHC health educators and administrators were asked to answer questions informed by the Consolidated Framework for Implementation Research (CFIR) and the Adoption, Implementation and Maintenance components of the RE-AIM program evaluation framework.20,21 Specifically, health educators were asked questions about the training they received, their experiences implementing the 2 program including challenges they experienced, and lessons learned. Administrators were asked about the value of the programs to their clinic and to patients, as well as program feasibility and sustainability.
Data Analyses
Quantitative Analysis
Descriptive statistics, χ2 tests, and 1-way ANOVAs were computed for baseline patient measures, initially testing for differences between: (1) intervention arms and (2) those with and without follow-up data available (see Table 1). To balance patient reported information while maximizing use of available data, participant self-reported smoking status and outcome data were used as the primary data source for smoking outcomes where available, and supplemented with EHR derived data where participant self-report data were not available. Data that were not available from either self-report or EHR for an individual were conservatively coded as “no quit attempt” and/or “continued smoking.” to reflect no change in smoking status or outcome. Linear and logistic regression analyses examined intervention group differences on smoking process and status outcomes. In addition to unadjusted models, models were adjusted for covariates based on baseline group differences and/or associations with outcomes from the extant literature (see Table 2).
Participant Characteristics by Intervention Group
Outcomes by Intervention Group (Missing Data Filled in with “Conservative Value” e.g., Did Not Quit Smoking)*
Logistic regression analyses further examined constructs associated with improvement in the 2 primary outcomes: (1) making ≥1 quit attempt and (2) smoking abstinence at follow-up for 7 or more days. Variables based on extent literature included in Step 1 included baseline: age, gender, race, daily smoker status, number of cigarettes per day, confidence in being smoke free in 6 months, and intervention group. Additional baseline variables of interest were examined in individual models in Step 2: education level, body mass index (BMI), stages of change, use of e-cigarettes/vaping, use of other tobacco products, and time to first cigarette (< vs ≥ 30 minutes). Number of clinical follow-up appointments (≥2 vs <2) and prescription of a nicotine product during the intervention period were examined in Step 3 in addition to variables in Step 1 and variables significant at Step 2 (P < .05), with variables from Step 2 and 3 reaching statistical significance included in a final multivariate model. Finally, potential interactions between intervention group and variables in Steps 1 to 3 were selected a priori and explored for any differential pattern of associations with the primary smoking outcomes. (see Table 3)
Logistic Regressions for Primary Outcomes*
Qualitative Analyses
Patient participant, health educator, and clinic administrator interviews were audio recorded, transcribed with removal of identifiers, and analyzed using research tools available at dedoose.com. Three researchers (MP, JT, KY) participated in independent review of the transcripts, with at least 2 members of the research team reviewing and taking notes on each individual transcript. Using an iterative process, emergent themes from each group of interviews were identified, classified, and summarized across the prespecified domains of inquiry described above, according to methods described by Morgan and Nico.22
Results
Participant Characteristics
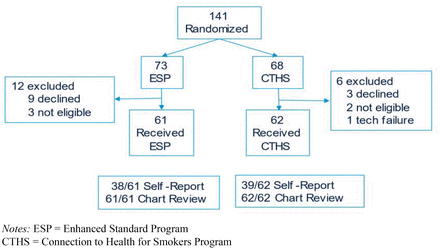
The CONSORT diagram is shown in Figure 1. 141 individuals attended a study enrollment visit and were randomized, with 12 declining participation after having the study explained to them, 5 determined to be ineligible after randomization, and 1 who could not be enrolled due to problems with internet connectivity. A total of 123 participants completed enrollment and met with a health educator. While 71 (58% of 123) participants completed the 3-month survey follow up assessment, we were able to review medical records of all 123 participants for evidence of primary care follow up during which smoking was addressed, which was included in our final analyses when necessary to complement other available outcomes data.
Study flow diagram.
Demographic characteristics are displayed in Table 1. At baseline, most participants in both groups described themselves as daily smokers (78.2%), smoking at least 10 cigarettes on days they smoked (mean = 11.7 cigarettes, S.D. = 7.2 and having smoked for > 20 years (64.9%). A minority reported using other nicotine products at baseline (12.3% e-cigarettes/vaping; 20.3% other tobacco products such as cigars, or chewing tobacco), in addition to cigarette smoking. While the mean confidence of being smoke free for at least 30 days in the next 6 months was only moderate within each group (mean = 6.0 on a 0 to 10 scale, S.D. = 3.1), most participants (81.3%) indicated that they were in the process of preparing or attempting to quit within the next 30 days.
As part of CTHS, we collected additional information on social and behavioral determinants of health. 39 (64%) experienced general life stress in the last week, 30 (48%) reported fair or poor health, and 24 (39%) reported 2 or fewer social close contacts in the last week. In addition, 14 (23%) reported current food insecurity, 9 (15%) reported not feeling safe in their community, 7 (12%) reported unstable housing, 7 (12%) had foregone health care due to lack of transportation. 12 (19%) reported a current history alcohol binge drinking, 12 (19%) reported illegal drug use and/or prescription drug misuse during the last year.
CTHS Action Plans
In the CTHS Arm, the health educator worked collaboratively with each participant to select an appropriate action plan based on their social circumstances and readiness to quit. Of the 62 CTHS participants, 40 (65%) chose a “Quit Plan,” 19 (31%) chose a “Change the Way I Smoke Plan” and 3 (5%) chose an alternative action plan not directly related to smoking habits. Among those choosing a Quit Plan, 34 planned to try medications, 32 planned to ask for support from friends and family, and 19 planned to use other resources such as the California Smoking Quitline or smokefree.gov. 35 were able to identify specific feelings, habits, and/or social situations that trigger a desire to smoke, and 34 were able to select specific strategies to address 1 or more of their triggers in real time. 11 opted to receive at least 1 text reminder to help prepare for their quit date, and 19 opted to receive between 1 and 3 daily text reminders with a motivational message of their choosing. Among those choosing a Change the Way I Smoke Plan, 14 planned to cut smoking by at least half, 7 planned to delay their first cigarette of the day, 4 planned to stop smoking when in the car, and 3 chose other goals. To achieve their goals, 5 planned to try medications, 14 planned to ask for support from friends and family, and 10 planned to use other resources such as the California Smoking Quitline or smokefree.gov. 12 were able to identify specific feelings, habits, and/or social situations that trigger a desire to smoke, and 13 were able to select specific strategies to address 1 or more of their triggers in real time. 11 opted to receive between 1 and 3 daily text reminders with a motivational message of their choosing. 4 of the 19 participants in this group selected the Change Plan after originally starting out in the Quit Plan group. The 3 participants who chose an alternative action plan unrelated to smoking chose instead to work on diet and/or exercise.
Outcomes by Intervention Group
We report outcomes by intervention group in Table 2. In both study arms, the majority of participants attended a follow up appointment during the study period with more CTHS than ESP participants reaching the threshold of ≥ 2 completed follow-up appointments (41.0% vs 62.9%, adjusted P = .01). Likewise, 2-thirds of the sample was prescribed a nicotine product with a nearly significant group difference with higher frequency in the CTHS group (74.2% vs 60.7%; adjusted P = .08). The majority of participants made at least 1 quit attempt (58.1%) and most reported cutting back on the number of cigarettes smoked per day (60.2%) with no difference between intervention groups. At final follow-up, 41 (33.3%) participants reported or were documented as not smoking in the last 24 hours, and 23 (18.7%) reported or were documented as not smoking in the past 7 days. ESP participants were more likely to have quit smoking for at least 7 days (24.6% vs 12.9%; adjusted P = .03).
Multivariate logistic regression analyses presented in Table 3 indicate that baseline patient reported level of confidence in becoming “smoke-free” within 6 months was associated with greater likelihood of quit attempts during the study period (P = .02) and that being prescribed any nicotine product or being assigned to the ESP group was more likely to lead to a report of 7-day abstinence at follow up (P = .04 for both outcomes). Though abstinence was determined by self-report, we did perform carbon monoxide breath testing on 22 participants who came in for in person follow-up, and all 7 who reported not smoking were confirmed to have carbon monoxide breath test result of 7 ppm or less, verifying their self-report. In exploratory analyses we examined interactions between predictors and intervention group for the primary outcomes, and no significant interaction was detected.
Structured Qualitative Interviews with Patient and Staff Participants
We interviewed clinic administrators at each site who were directly responsible for supervising the health educators. We interviewed health educators at each site with experience administering each program to at least 5 patients. We interviewed individuals in both arms until reaching saturation. Qualitative interviews with clinic administrators and health educators focused on program feasibility and acceptability, as well as perceived differences between ESP and CTHS and suggested program improvements. Qualitative interviews with patients focused on their overall experience with the program to which they were assigned, including perceptions of program content and level of support they received to succeed with their goals. These findings are summarized in Table 4.
Summary of Qualitative Interviews with Clinic Administrators (n = 5), Health Educators (n = 5), and Patients (n = 31) Regarding Enhanced Standard Program (ESP) and Connection to Health for Smokers Program (CTHS)
Discussion
Both ESP and CTHS enabled CHC clinical teams with minimal experience to identify and support patients in various stages of readiness to quit in the process of smoking cessation. Clinic administrators and health educators indicated that both programs aligned well with their clinical priorities and workflows, and that they could be further strengthened through full integration within the EHR. The 2 programs were perceived as having different strengths. Health educators appreciated the ease of implementation for ESP, while CTHS was perceived as more holistic and individually tailored to social and behavioral needs. Patients in both programs reported high levels of satisfaction. Those receiving ESP appreciated the education, though some felt that the education provided was not new to them. CTHS patients appreciated the detailed action planning process even though following through on these plans was not always successful. In both programs, patients indicated desire for continued engagement and support over a longer period time. The positive reception of both programs should encourage CHCs consider delivering comprehensive on-site smoking cessation programs for patients who may need more than a referral to a quit line or who may have difficulty accessing off-site programs and services.
There are several possible reasons why ESP appeared to perform better on the main outcome of successful smoking cessation than CTHS. First, ESP was focused solely on quitting smoking, whereas CTHS assessed and addressed other social, behavioral considerations in parallel with the topic of smoking cessation. It may be that smoking cessation is more successfully addressed with a narrower focus and without attempting to tease out social or behavioral health needs before providing the 5 As of smoking cessation.23,24 Second, as health workers received trainings and delivered both programs to patients, contamination between intervention arms may have occurred. It is likely that health workers borrowed learnings and approaches from both programs when counseling patients in each arm. ESP included more unstructured time than CTHS, making it especially likely that elements of CTHS were delivered to many ESP patients. In fact, our field observations and postintervention key informant interviews indicated that contextual factors were often addressed by health educators in both study arms, with referrals to food programs, substance use counselors, and social workers in many cases.
Another limitation is that the pragmatic design of the study did not allow the study team to survey ESP patients about their social and behavioral barriers to smoking cessation to compare the groups. However, the randomization process produced 2 groups that were similar based on other demographic characteristics. Sample size was by design limited in this initial feasibility study only allowing for power to detect medium effects (d = 0.51) or greater and null findings should be interpreted in the context of the sample size. In addition, in person follow up was incomplete in both arms, and data abstraction was limited by chart documentation. Incomplete documentation of smoking status in medical records is common across health systems.25 Nevertheless, we confirmed many successful outcomes in both arms.
In summary, this study shows that comprehensive smoking cessation programs can be delivered in CHCs where a large proportion of smokers receive primary care. Furthermore, CHC health workers can become effective counselors when supported with structured programs. Patients who are motivated to quit smoking but might not be able or willing to engage in smoking cessation activities outside of primary care may benefit from participation in these programs. While it remains uncertain if an awareness of social and behavioral challenges enhances the effectiveness of smoking cessation action plans in these settings, an awareness of such challenges can assist CHC health workers to identify and simultaneously address health related concerns with potential short- and long-term benefits for patients.
Acknowledgments
The authors would like to acknowledge Contra Costa County Health Services, whose medical staff, nursing staff, and patients participated in program development, implementation, and evaluation of this research program.
Notes
This article was externally peer reviewed.
Funding: This research was funded by the California Tobacco-Related Disease Research Program (27IP-0024). Additional research infrastructure support was provided by the National Center for Advancing Translational Sciences, through the UCSF Clinical and Translational Sciences Institute and the San Francisco Bay Collaborative Research Network (UL1 TR001872).
Conflict of interest: None.
To see this article online, please go to: http://jabfm.org/content/37/1/84.full.
- Received for publication June 24, 2023.
- Revision received August 10, 2023.
- Accepted for publication August 21, 2023.