Abstract
Background: Evidence supports the clinical effectiveness of intimate partner violence (IPV) screening programs, but less is known about implementing and sustaining them. This qualitative study identified implementation strategies used to integrate IPV screening programs within Veterans Health Administration (VHA) women's health primary care.
Methods: Thirty-two administrators and clinician key informants from 11 VHA facilities participated in semistructured interviews. Implementation strategies were identified using established definitions from implementation science literature, through multistep content analysis, involving site comparisons by implementation status.
Results: We identified 8 implementation strategies. Three were present across all sites: (1) conduct ongoing IPV trainings, (2) conduct educational meetings and outreach visits, and (3) develop and distribute educational materials. Five strategies were unique to early adopting sites: (4) identify and prepare champions, (5) change record systems to remind clinicians, (6) create a learning collaborative through advisory boards or workgroups, (7) audit and provide feedback with relay of clinical data to providers, and (8) access new funding.
Discussion: Strategies align with and extend literature addressing barriers to screening. Evidence shows that effective IPV screening implementation in primary care requires a bundle of well-defined, carefully selected strategies.
Conclusions: Implementation strategies used collectively can enable integration of IPV screening programs in primary care.
- Implementation Science
- Intimate Partner Violence
- Primary Health Care
- Qualitative Research
- Veterans Health Administration
- Women's Health
Intimate partner violence (IPV) is a substantial threat to women's safety and health.1 Although individuals of any gender identity can experience IPV, women are disproportionately affected, with elevated prevalence, severity, and health impacts.1 Within the United States, at least 1 in 4 women experience physical violence, sexual violence, or stalking by a partner that results in health-related impacts (eg, fear, safety concerns, injury, psychological symptoms, and need for medical care).2 This troubling prevalence estimate does not include psychological IPV, which has particularly adverse effects on health.3,4 IPV is associated with higher health care use, including higher primary care use.5,6 Supported by evidence from a systematic review,7 the US Preventive Services Task Force recommends IPV screening, counseling, and offering referrals during primary care visits for women,8 a cluster of practices referred to as “IPV screening programs.”9 Such programs enable primary care providers (PCPs) to offer emotional and tangible support, resources, and referrals to services that can enhance safety and health.10 But implementing any new clinical practice or changes to clinical practice is challenging, particularly for a complex, stigmatized public health problem like IPV.11
Despite the proliferation of IPV curriculum,12 few states in the United States currently require IPV screening continuing medical education,13 and the level of PCP knowledge about IPV identification and referral for patients who endorse IPV is modest and highly variable. In fact, only 2% to 50% of all health care providers report screening for IPV on a regular basis.14 With limited provider education and personal discomfort reported as major barriers to screening,13,15⇓⇓–18 IPV training for PCPs is critical. Provider educational efforts are important implementation strategies in response to this gap; however, training alone is not sufficient for sustained adoption. Provider education is associated with short-term increases in provider screening rates, but studies suggest its insufficiency, as provider-education-focused implementation efforts show no significant long-term effect on screening uptake or identification rates over time.19
In addition, there are common logistical barriers such as time constraints, competing priorities within visits, and lack of referral options for counseling or support services.13,16,18,20 Additional implementation strategies used to promote IPV screening include (1) giving providers screening guidelines or protocols,19 (2) placing screening prompts for providers in patients' health records 21 or changing the record system to provide reminders in patients' health records,22⇓⇓–25 (3) having a designated victim advocate onsite,19 (4) involving executive boards 24 or stakeholder groups,26 and (5) soliciting feedback from staff and other clinic representatives.26,27 However, it is unclear from the literature how effective each of these strategies is on their own or when used in combination. Thus, it remains unknown what package of implementation strategies is needed for effective and sustainable implementation of IPV screening programs.
The integration of implementation science into IPV screening research and evaluation can help close this gap.28⇓–30 Within the implementation science literature, the Expert Recommendations for Implementing Change (ERIC) project31 established a set of 73 clearly conceptualized implementation strategies to guide consistent definitions in research and practice. Although ERIC has been used for understanding the implementation of other health care interventions (eg, see Perry et al32), there are no published studies assessing strategies for successful IPV screening implementation purposefully using the ERIC compilation. There remains a need to identify key implementation strategies for integrating IPV screening programs in primary care, using conceptually clear and consistent definitions.33
IPV is a critical issue for the Veterans Health Administration (VHA),34 the largest integrated health care system in the nation, as nearly 1 in 5 (18.5%) women using VHA primary care have experienced past-year IPV.6 As VHA started implementing IPV screening programs in primary care in 2014, as recommended by the VA Domestic Violence/IPV Task Force,35 there is an opportunity to learn about implementation strategies used in this context. Specifically, the use of discrete implementation strategies across VHA Medical Centers (VAMCs) in attempts to integrate IPV screening programs remains unknown.
This study uses the ERIC compilation of implementation strategies31 to identify discrete implementation strategies used at VAMCs to implement IPV screening programs in primary care clinics across the country, through a qualitative study with VHA health care providers and administrators. Unlike prior research that mostly looks at controlled IPV screening trials and interventional studies,9 the current study focuses on naturally occurring strategies used in VHA. Our goal is to document implementation strategies collectively used for implementation of routine IPV screening across VHA primary care clinics. Findings can inform the spread of IPV screening programs across VHA as well as implementation strategies for other integrated health care systems.
Method
Participants
With study approval from the Institutional Review Board of the VA Boston Healthcare System, we recruited 11 VAMCs nationwide, at varying stages of IPV screening program adoption in primary care. From these sites, we enrolled and interviewed 32 key informant clinicians and administrators with knowledge of or involvement with IPV screening and response programming in their local women's health primary care clinics (eg, women's health medical director, PCP, primary care social worker, IPV Assistance Program coordinator, women veterans program manager). Additional details regarding participants and recruitment procedures are published elsewhere.20
Procedures
Sites were selected using purposive sampling36 based on program evaluation findings from our operational partners in VHA's Office of Women's Health Services. Women's health primary care staff completed brief surveys regarding current adoption status. Early adopting sites were defined as those that reported currently engaging in IPV screening as part of routine care (ie, screening all female patients of childbearing age or all female patients at least annually). Late-adopting sites were defined as those that reported not currently engaging in IPV screening as routine care (ie, screening at provider's discretion).20 We recruited and enrolled 6 early adopting sites and 5 late-adopting sites for this study. Sites were geographically spread across the United States.
The research team, which included experts in IPV, trauma-informed primary care, qualitative research, and implementation science, developed a semistructured interview guide based on the integrated-Promoting Action on Research Implementation in Health Services (i-PARIHS) framework.37 i-PARIHS provided a conceptual framework to characterize the ways in which IPV screening practices and implementation strategies have been used in VHA. Questions focused on understanding the history of IPV screening program implementation efforts at the site. Sample questions included: “How was IPV screening initially started at your primary care clinic(s) and who was involved in that?”, “What helped you to implement IPV screening or what has made it easier?”, and “What have been some of the challenges and what did your facility do to overcome them?” Questions were designed to be flexible, and the interviewer asked follow-up questions and probed for examples to understand strategies used to support implementation.
Data Collection
Between October 2017 and February 2018, the last author conducted all one-on-one, semistructured phone interviews with 32 key informants across 11 sites. As much as possible, participants were asked the same questions (using the interview guide) regardless of their site's IPV screening adoption status. Interviews were recorded with participants' permission and transcribed verbatim.
Data Analysis
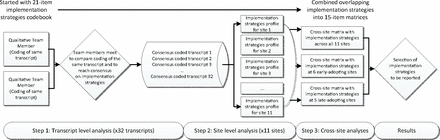
Rapid content analysis38 was applied to identify implementation strategies used at study sites. We created a codebook based on the refined compilation of implementation strategies from the ERIC project.31 This compilation provides a comprehensive list of implementation strategies that can be used in isolation or combination to support implementation efforts,31 our team selected 20 strategies a priori most relevant to IPV screening program implementation and adapted their definition accordingly (eg, replacing “clinical innovation” by “IPV screening”). The code “other implementation strategies” was also added to inductively capture strategies beyond those identified deductively, resulting in a 21-item codebook used for analysis. Four team members trained in qualitative approaches then independently coded 4 transcripts using the deductive codebook, convened to discuss and refine codebook definitions, and reached consensus across codes and transcripts.38 Thereafter, each of the 28 remaining transcripts was coded by 2 team members with ongoing consensus meetings. Data analysis steps are summarized in Figure 1.
Qualitative data analysis steps.
Step 1: Transcript-Level Analysis
Our team reviewed each transcript against the 21-item codebook, selecting illustrative quotes for each identified strategy. A strategy could receive multiple codes. Each transcript was coded by 2 team members separately, who then met routinely for an hour on average and discussed discrepancies to reach consensus.
Step 2: Site-Level Analysis
As each site had 2 to 4 participants (32 participants across 11 sites), consensus codes from transcripts were merged to create each site's implementation strategies profile on an Excel spreadsheet. Each row represented a strategy, while 3 columns contained the codebook definition, summary evidence, and illustrative quotes for that site.
Step 3: Cross-Site Analysis
We combined site profiles into a cross-site Excel matrix where 21 rows listed all implementation strategies codes, and 11 columns summarized each site's evidence per strategy. On cross-site matrix review, an empty row labeled “provide clinical supervision” was removed due to lack of evidence at any site. In performing matrix content analysis, we observed overlap of evidence across several strategies, consistent with the interconnectedness found between ERIC-based strategies in prior screening trials.32 Hence, we combined 5 of the 20 rows with the remaining 15, and tailored strategy definitions and evidence accordingly. For example, “audit and provide feedback” was combined with “facilitate relay of clinical data to providers,” because the team agreed that available evidence for both strategies was the same per their respective definitions. Similarly, overlaps of evidence between other categories, including: (1) “conduct educational meetings” and “conduct educational outreach visits”; (2) “change record systems” and “remind clinicians” to screen for IPV; (3) “create a learning collaborative” and “use advisory board or workgroups”; and (4) “develop IPV education materials” and “distribute IPV educational materials” led to additional categorical merging.
The final cross-site matrix from which findings were derived had 15 refined implementation strategies across all sites. We created 2 submatrices to examine strategies across early adopting and late-adopting sites, separately.
Results
Our analysis highlighted 8 implementation strategies, each evidenced by 3 or more sites in our 11-site sample. These strategies and definitions are displayed in Table 1. We categorized them into 2 groups: (1) strategies present across sites regardless of IPV screening program adoption status, and (2) strategies present only in early adopting sites. We found no strategies unique to late-adopting sites. Table 2 displays exemplar quotes for all 8 implementation strategies. It is also notable that while early adopting sites each used on average 4.25 of these 8 implementation strategies, late-adopting sites engaged with 2.67 such strategies on average (see Table 3).
Definitions of Intimate Partner Violence Screening Implementation Strategies Used
Intimate Partner Violence Screening Implementation Strategies with Exemplar Quotes for Strategies Across Early and Late-Adopting Sites, and Early Adopting Sites Only
Implementation Strategies Across Sites by Intimate Partner Violence Screening Adoption Status
Implementation Strategies across All Sites
Three strategies were common across early and late-adopting sites.
Conduct Ongoing IPV Trainings
Ongoing trainings that built on each other over time enabled providers (eg, physicians, nurses, social workers) to effectively integrate IPV screening practices. In-depth trainings were various combinations of basic IPV education: tips on talking about IPV with patients using trauma-informed care principles; roleplays to help providers gain firsthand experience with screening and responding and problem-solving potentially challenging situations; clarifying clinical pathways following disclosures; how to offer education, validation, and support; and resource options. Trainings were presented in different formats: regular dedicated training events, trainings incorporated into larger staff events, retraining where deficiencies in screening delivery were identified, and additional one-on-one trainings to fill gaps outside of scheduled training events.
Conduct Educational Meetings and Educational Outreach Visits
Sites discussed intermittent “bite-sized” IPV screening education delivered to other relevant providers and stakeholders. IPV-trained staff (eg, psychiatrist, social worker) used events such as town hall meetings or discipline-specific events (eg, primary care leadership meetings) to deliver IPV screening education in various formats (ie, lectures, case examples, and art projects). These efforts included traveling to external locations such as community-based outpatient clinics in both rural and urban settings to share this knowledge. Educational information included IPV statistics and context to enhance provider knowledge and buy-in. Educational meetings and educational outreach visits differed from ongoing IPV trainings. Such meetings were not ongoing. They focused on the delivery of easily grasped information to increase awareness of IPV and screening recommendations. In contrast, conducting ongoing IPV trainings speaks to the repetitive, continuous nature of in-depth knowledge and skill transfer to improve clinical practice.
Develop and Distribute IPV Educational Materials
Sites either created or adapted materials used for staff and providers' IPV training (eg, presentations, handouts); compiled, maintained, and shared lists of community resources on IPV with providers; and developed screening protocols and documentation guidelines for teams to use when screening patients for IPV. Materials were distributed in the facility either electronically through e-mail and SharePoint (eg, PowerPoint slidedeck that departments can tailor to fit their needs), via flyers posted in common areas, or in person (eg, training sessions, PCPs giving patients brief handouts or brochures).
Implementation Strategies across Early Adopting Sites Only
Five implementation strategies were present only in early adopting sites and are described next.
Identify and Prepare Champions
For all early adopting sites, having dedicated IPV champions was essential to implementation. Champions' dedication to driving IPV screening implementation manifested through: (1) regular participation in national calls/meetings led by VHA leadership to access a wider network of resources; (2) relationship building with the community (ie, meetings, summits) and gaining access to community networks and resources; (3) training and educating staff and providers; (4) champions in leadership promoting a clinical reminder to facilitate screening; and (5) champions conducting IPV screening embedded within the clinic for efficiency, tailoring the process to local contextual needs.
Change Record Systems to Remind Clinicians
Most sites used electronic medical records systems to remind clinicians, thus facilitating adoption of, and comfort with, IPV screening practices. Specifically: (1) clinical reminders prompted routine IPV screening and response practices; (2) screening electronically made it easier for PCPs to record screening results, additional assessments, and clinical resources provided; (3) the ability to turn on the clinical reminder for only some PCPs made piloting and refining IPV screening protocols possible without having to train all hospital PCPs beforehand; and (4) tracking positive screens created useful data to refine the screening process.
Create a Learning Collaborative through Advisory Boards or Workgroups
Most sites created groups of stakeholders involved in IPV screening program implementation, giving/receiving support and advice, and sharing knowledge. Some local workgroups (ie, advisory board, committee, taskforce) had stakeholders from different areas (ie, IPV experts, medical university staff, social workers, veterans). They met regularly to coordinate IPV screening-related tasks (ie, educational events), develop relationships with relevant areas of the hospital, and problem-solve challenges. In addition, they called in external experts from the community to aid in assessment of IPV and community referrals. Sites that were further along played a mentoring role to sites in the process of implementation. Lastly, access to a broader network provided opportunities for more informal connections and collaborations, serving both to implement and, when relevant, sustain IPV screening programs.
Audit and Provide Feedback, with Relay of Clinical Data to Providers
Most sites collected and analyzed data on their process to promote IPV screening uptake and optimization, all the while informing PCPs on program implementation progress and areas for growth. Concretely, these data were used to demonstrate the need for an IPV Assistance Program coordinator or a clinical reminder, show screening deficiencies, assess IPV screening success, and determine next steps. Sites already using electronic clinical reminders for IPV screening could more easily monitor and share program performance.
Access New Funding
A few sites secured new or existing money from VHA leadership to facilitate the implementation of IPV screening. Funding included grants to create a clinical reminder and distribute resources to patients who endorsed IPV and a dedicated IPV Assistance Program coordinator position to protect time and resources for supporting IPV screening program implementation and sustainment.
Discussion
Evidence supports the clinical effectiveness of IPV screening programs, but implementation in real-world care is challenging. This study identifies 8 implementation strategies used to support IPV screening program implementation in the context of VHA primary care.
It is not surprising that 3 of these strategies, evidenced across early and late-adopting sites (ie, conduct ongoing IPV trainings, conduct educational meetings and educational outreach visits, and develop and distribute IPV educational materials), focus on training and education with adequate supporting materials. This is consistent with literature identifying provider education and personal discomfort with IPV as primary barriers to IPV screening.14,19 Identified implementation strategies pertaining to developing and distributing educational materials (eg, sharing a list of community resources with providers to enable tailored referrals) also align with prior literature indicating that adding clinical tools and guidelines (eg, screening protocols, referral lists) to education efforts tends to improve the implementation and clinical effectiveness of IPV screening programs.9 These foundational implementation strategies may be relatively more feasible and lower cost compared with other more intensive implementation strategies. Regardless, these 3 strategies seem to be a primer for all sites in propelling screening forward.
We identified 5 additional implementation strategies being used uniquely in early adopting sites. These strategies were likely selected to overcome specific barriers confronted during the early stages of implementation at these sites. These additional strategies align well with common barriers to IPV screening identified in prior research. They confirm the need for additional implementation strategies (beyond training and education) in addressing barriers to implementation; namely, (1) identifying and preparing champions, including social work and mental health providers with specific expertise in IPV and who can help identify clinical pathways following disclosures;20,39 (2) auditing and providing feedback with relay of clinical data to providers; and (3) accessing new funding, collectively heightening the perceived importance of IPV screening programs.20,40 In addition, (4) changing record systems to remind clinicians helps mitigate time constraint;13,16,18,20 and (5) creating a learning collaborative through advisory boards or workgroups ensures widespread understanding of the impact of IPV on women's health.13,30 These 5 strategies are also concordant with strategies used to complement providers' IPV education as noted in prior literature (eg, having an onsite clinician to provide follow-up services, involving executive board or stakeholder groups, and eliciting staff feedback).21⇓⇓–24,26,27 Although late-adopting sites had not used these strategies at the time of this study's data collection, it is possible that these sites eventually used one or more of these strategies as their implementation efforts continued. Additional site-specific barriers likely contributed to the selection of strategies at different sites.
The fact that early adopting sites used these 8 implementation strategies (average 4.25 strategies per early adopting site compared with 2.67 strategies per late-adopting site) suggests that multiple implementation strategies used in tandem facilitate routine IPV screening and response practices. It is also likely that contextual factors, such as resources and number of providers in the clinic, contributed to successful adoption. Indeed, there were indicators that early adopting sites had greater staffing and capacity (eg, IPV champion in place and grant funding) than late-adopting ones, which may have contributed to greater on-site IPV screening expertise.
This study has identified strategies used collectively to implement IPV screening programs in VHA primary care. Strengths include the use of an established implementation science framework and methods. The use of ERIC to identify implementation strategies extends the IPV screening literature and was feasible with tailoring of the definitions to our study. This included (1) allowing multiple codes for a strategy when appropriate, (2) using a manageable number of codes (eg, selecting 20 out of 73 strategies available), and (3) combining overlapping codes at the analysis stage. These findings could inform the establishment and testing of a comprehensive toolkit of implementation strategies for IPV screening implementation across VHA primary care settings, to which the present 8 strategies are a start.
This study has several limitations. This study was conducted within VHA, and it is possible that these strategies may not be adequate in other settings, especially in nonintegrated care contexts or those without IPV clinical experts. The IPV Assistance Program coordinators are fairly unique to VHA, and they were often the most knowledgeable about implementation efforts. Findings may not translate well to smaller health care systems and practices with a narrower scope that do not have the availability of an on-site IPV consultant. However, the expansion of mental/behavioral health services within primary care clinics offers opportunities for enhancing IPV detection and care. Another limitation is that other implementation strategies not subsumed within our selected a priori codes from the ERIC compilation would not be captured in this study. Therefore, it is possible that other important implementation strategies used across early and late-adopting sites were not identified. However, strategies identified under “other implementation strategies” were minimal, signaling this as a minor limitation. Future studies could use surveys to assess for ERIC-based implementation strategy use in IPV screening implementation efforts.
Successful integration of IPV screening programs within routine care is critical in addressing the public health burden of IPV. Understanding promising implementation strategies used in previous IPV screening efforts in VHA is timely. As of January 2019, VHA policy requires IPV screening as a standard of care.41 VHA has already initiated a larger-scale effort to spread IPV screening programs nationwide using the strategies highlighted in this study with a complementary mixed-method evaluation of the implementation impact.30 Resulting knowledge will further guide effective IPV screening implementation in health care, beyond VHA primary care. Done right, routine IPV screening can significantly reduce IPV-related adverse outcomes to women's health.
Acknowledgments
Our special thanks to Rachel M. Maskin for her assistance with data analysis, Cassidy Gutner, PhD, for her comments on the original study design, and to the clinicians and administrators who shared their perspectives for this research. We also thank VHA's Office of Women's Health Services and the VHA IPV Assistance Program of Care Management and Social Work Services for providing program evaluation metrics for sampling and recruitment.
Notes
This article was externally peer reviewed.
Funding: This research was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development (HSR&D) Services (PPO 17-044; PI Iverson). This work was also supported, in part, by KMI's Presidential Early Career Award for Scientists and Engineers (USA 14-275) and Implementation Research Institute fellowship from the National Institute of Mental Health (5R25MH08091607) through HSR&D Services, Quality Enhancement Research Initiative.
Conflict of interests: None declared.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
To see this article online, please go to: http://jabfm.org/content/34/2/346.full.
- Received for publication June 24, 2020.
- Revision received September 4, 2020.
- Accepted for publication September 20, 2020.