Abstract
Inverse psoriasis is a disorder of intertriginous areas of the skin that can easily masquerade as candidal intertrigo. Candidal rashes are commonly encountered in primary care and typically respond promptly to therapy. When treatment fails, nonadherence to treatment and medication resistance often are suspected; however, the possibility of an incorrect diagnosis should also be entertained. This article presents the case of a patient with inverse psoriasis who was misdiagnosed with recurrent candidal intertrigo multiple times. The diagnosis and treatment of inverse psoriasis is reviewed, and other conditions that may be confused with Candida and inverse psoriasis, including bacterial intertrigo, tinea, and seborrheic dermatitis, are discussed. When confronted with a case of “resistant Candida,” consideration of inverse psoriasis and other Candida mimics can allow physicians to diagnose and treat these conditions more effectively, avoiding the frustration experienced by our patient.
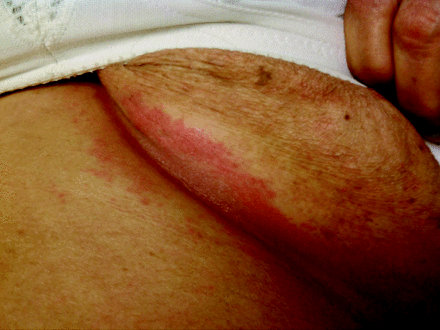
A 68-year old woman with a history of poorly controlled type 2 diabetes mellitus presented as a new patient with exacerbation of chronic obstructive pulmonary disease (COPD) and a rash. The rash consisted of chronic, recurrent, erythematous patches in the inframammary and inguinal folds (Figure 1). The patches were mildly macerated. Scanty satellite lesions were observed near the inframammary but not the inguinal patches; scales were absent in these locations. There was scaling and tenderness in both auditory canals, with mild scaling of each pinna. Over the past week, the patient had applied multiple topical medications to the rash, including clotrimazole, moisturizer, and bacitracin/neomycin/polymyxin. She reported a 10-year history of similar rash affecting the axillae, groin, inframammary folds, retroauricular area, and gluteal cleft. The skin lesions had been diagnosed previously as candidiasis, but the patient was adamant that multiple trials with topical and oral antifungals did not help the rash. It is interesting that she noted that the skin lesions always improved when she was taking prednisone for the treatment of COPD exacerbations.
Erythematous patch in the inframammary fold.
The patient was prescribed prednisone for her COPD exacerbation and neomycin-polymyxin B-hydrocortisone otic solution for presumed otitis externa. Antifungal cream was offered but the patient was certain it would not help and insisted the prednisone would resolve the rash. She was instructed to discontinue all topical products for the rash and to return for follow-up. She presented 1 week later with a dramatic improvement in her rash. However, within 2 weeks of terminating oral prednisone, the erythematous patches recurred in the inframammary folds and gluteal cleft. The differential diagnosis included intertrigo, erythrasma, seborrheic dermatitis, inverse psoriasis, and resistant Candida secondary to poorly controlled diabetes. The affected areas were examined with a Wood's lamp and demonstrated no signs of fluorescence that would suggest erythrasma. A fungal culture was obtained, and clotrimazole-betamethasone dipropionate topical cream was prescribed to cover both fungal and psoriatic etiologies. Four weeks later, the fungal culture showed no growth and the rash had improved. The lack of growth on the fungal culture and resolution of the rash with oral and topical steroids suggested a diagnosis of inverse psoriasis. To avoid skin atrophy in intertriginous areas from prolonged steroid use, the patient was prescribed topical tacrolimus for exacerbations.
She had a good response to tacrolimus initially. When this response began to wane, she was referred to dermatology, where a diagnosis of inverse psoriasis was confirmed. A daily regimen of topical tacrolimus was initiated, with desonide ointment prescribed for flares. The rash was well controlled at follow-up 3 months later.
Discussion
Inverse psoriasis is considered an anatomic variant of psoriasis rather than a separate entity. It has not been assigned a distinct code in the International Classification of Diseases, Ninth Revision, making the true incidence of inverse psoriasis difficult to determine. However, the worldwide prevalence of psoriasis is approximately 1% to 3%,1 and recent studies suggest that 3% to 12% of psoriatic patients manifest inverse psoriasis.2⇓⇓–5 Although not particularly common, inverse psoriasis can mimic several conditions often encountered by primary care physicians. Thus it is an important diagnosis for the family physician to consider when evaluating a patient who presents with an erythematous rash in an intertriginous area.
Plaque psoriasis is the most common form of psoriasis. It is characterized by red, sharply demarcated papules or plaques with overlying, adherent, silvery-white scales that bleed when removed. Plaque psoriasis typically affects the scalp, sacrum, and extensor surfaces of elbows and knees in a symmetric pattern.1 In contrast, inverse psoriasis occurs in flexural areas such as the retroauricular area, axillae, inframammary creases, inguinal folds, and intergluteal cleft.6 As a consequence, inverse psoriasis may also be referred to as “flexural psoriasis” or “intertriginous psoriasis.” The lesions of inverse psoriasis are erythematous and sharply demarcated, as in plaque psoriasis, but scales are typically absent. The surface is smooth, moist, macerated, or all three and may contain fissures.7 Inverse psoriasis may be malodorous, pruritic, or both.1,7,8 If present, psoriasiform lesions elsewhere on the body, a family history of psoriasis, and characteristic nail abnormalities support a diagnosis of inverse psoriasis.2,9 The most common nail disorders observed in psoriatic patients include oil spots, pitting, subungual hypertrophy, and onycholysis.6
Inverse psoriasis is easily mistaken for infectious dermatoses, particularly bacterial or fungal intertrigo.10,11 Intertrigo is inflammation of opposed skin folds caused by skin-on-skin friction that presents as erythematous, macerated plaques. Secondary bacterial and fungal infections are common because the moist, denuded skin provides an ideal environment for growth of microorganisms. Candida is the most common fungal organism associated with intertrigo.9 Intertiginous candidiasis presents as well-demarcated, erythematous patches with satellite papules or pustules at the periphery.11 The presence of peripheral satellite papules or pustules can help differentiate intertriginous candidiasis from inverse psoriasis. Potassium hydroxide examination of skin scrapings should be performed if Candida is suspected because pseudohyphae confirm the diagnosis of candidiasis.8,9 Two studies have demonstrated an absence of Candida in inverse psoriasis lesions.12,13 We did not identify any studies that showed evidence of concomitant candidiasis and inverse psoriasis.
Bacterial species that often complicate intertrigo include Staphylococcus aureus, group A β-hemolytic streptococcus, Pseudomonas aeruginosa, Proteus mirabilis, and Proteus vulgaris. Cutaneous erythrasma, caused by Corynebacterium minutissimum, presents as red-brown macules that can coalesce into patches with well-defined borders.9 This too may mimic inverse psoriasis and Candida. Culture and sensitivity should be obtained if bacterial infection is suspected. Examination with a Wood's light can help identify P. aeruginosa (green fluorescence) and C. minutissimum (coral red-fluorescence).9 S. aureus has been shown to colonize psoriatic skin lesions, and a small number of case reports describe group A β-hemolytic streptococcus infection inducing guttate psoriasis.14 Therefore, a positive bacterial culture does not necessarily rule out underlying inverse psoriasis. This is an important consideration in suspected bacterial intertrigo that is unresponsive to treatment.
Tinea corporis and tinea cruris, both dermatophytic fungal infections, also have a clinical presentation similar to inverse psoriasis and Candida. Tinea corporis presents with a raised, annular border that may be active, with pustules or vesicles present; central scales may be appreciated in early lesions, whereas peripheral scales with central clearing is suggestive of more advanced lesions.1 Tinea cruris often manifests as well-demarcated, erythematous plaques with central clearing and elevated, scaling borders that may be active, with pustules or vesicles present.15 The raised border and presence of pustules, vesicles, or scales can help differentiate tinea from inverse psoriasis. However, potassium hydroxide examination of skin scrapings that demonstrates branching hyphae is the reference standard for diagnosis of tinea.9 Fungal culture can confirm the genus and species,1 although culture techniques have a limited role in evaluation because of the expense and time requirements.15
Seborrheic dermatitis is another cutaneous disease that can resemble inverse psoriasis or Candida.6,9,10 Typical locations for seborrheic dermatitis include the scalp, eyebrows, eyelids, nasolabial creases, ears, chest, intertriginous areas, axilla, groin, buttocks, and inframammary folds.16 Seborrheic dermatitis of body folds presents as a sharply marginated, brightly erythematous eruption, often with erosions and fissures.11 The presence of yellow, greasy scales can help differentiate seborrheic dermatitis from inverse psoriasis, but these scales may be absent in flexural areas. Seborrheic dermatitis of the flexural folds is uncommon in children and usually suggests immunodeficiency.17 Skin biopsy may help differentiate seborrheic dermatitis from inverse psoriasis, with the former showing a “spongiform” appearance that is absent in psoriasis.18 Inverse psoriasis demonstrates the typical psoriasiform reaction, including parakeratosis, epidermal hyperplasia, and elongation of rete ridges.19,20
If inverse psoriasis is diagnosed, short-term treatment (2 to 4 weeks) should be initiated with low- to mid-potency topical steroids, such as betamethasone valerate. The frequency of application can be tapered and ultimately discontinued if the psoriasis improves. Frequent and long-term use of low-potency topical steroids in intertriginous areas can result in atrophy, striae, and telangiectasia. For continued therapy beyond 2 to 4 weeks, calcipotriene, pimecrolimus, or tacrolimus should be initiated, or the low-dose topical steroid can be used 1 or 2 times per week for maintenance therapy.21 More resistant cases, like the one described herein, may require combination therapy. Other therapeutic options include botulinum toxin22,23 and efalizumab,24 although evidence of their effectiveness is limited to case reports.
Conclusion
Inverse psoriasis may mimic candidal intertrigo. Features that suggest inverse psoriasis include history of a similar rash that did not respond to antifungal treatment, a family history of psoriasis, the presence of classic psoriatic lesions elsewhere, and psoriatic nail changes. The rash usually responds well to topical steroids, topical immune modulators, or both. To avoid skin atrophy, topical steroids should not be used too freely; the rash is typically chronic and occurs in areas at higher risk for skin atrophy.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
- Received for publication August 16, 2012.
- Revision received October 8, 2012.
- Accepted for publication October 10, 2012.