Abstract
Background: The initiation and timely completion of the human papillomavirus (HPV) vaccine in young women is critical. We compared the initiation and completion of the HPV vaccine among women in 2 community-based networks with electronic health records: 1 with a prompt and reminder system (prompted cohort) and 1 without (unprompted cohort).
Methods: Female patients aged 9 to 26 years seen between March 1, 2007, and January 25, 2010, were used as the retrospective cohort. Patient demographics and vaccination dates were extracted from the electronic health records.
Results: Patients eligible for the vaccine included 6019 from the prompted cohort and 9096 from the unprompted cohort. Mean age at initiation was 17.3 years in the prompted cohort and 18.1 years in the unprompted cohort. Significantly more (P < .001) patients initiated the vaccine in the prompted cohort (34.9%) compared with the unprompted cohort (21.5%). African Americans aged 9 to 18 years with ≥3 visits during the observation period were significantly more likely to initiate in the prompted cohort (P < .001). The prompted cohort was significantly more likely (P < .001) to complete the vaccine series in a timely manner compared with the unprompted cohort.
Conclusion: More patients aged 9 to 26 years initiated and achieved timely completion of the HPV vaccine series in clinics using an electronic health record system with prompts compared with clinics without prompts.
The Advisory Committee on Immunization Practices (ACIP) currently recommends routine vaccination of girls and boys aged 11 or 12 years with 3 doses of a human papillomavirus (HPV) vaccine.1 The HPV vaccine is administered intramuscularly as 3 separate 0.5-mL doses, with the second dose occurring 1 to 2 months after the first dose and the third dose occurring 6 months after the first dose. The vaccination series can be started beginning at age 9 years, with catch-up vaccination recommended between 13 to 26 years. The vaccine series was approved for boys in 2010 by the US Food and Drug Administration and in 2011 by the Centers for Disease Control and Prevention.2
National estimates of HPV vaccine uptake provided by the Centers for Disease Control and Prevention's National Immunization Survey reported 25.1% of adolescent girls aged 13 to 17 years initiated the vaccine series (≥1 dose) in 2007.3 Between 2008 to 2012, HPV vaccine initiation increased from 37.2% to 53.8% and HPV vaccine series completion (≥3 doses) increased from 17.9% to 33.4% among adolescent girls.3 During the same time period of increased initiation and completion of the HPV vaccine series, 84% of unvaccinated girls were missing one or more opportunities to get the vaccine in 2012.3 Uptake has been substantially lower among adult women, with available data from the National Immunization Survey–Adult indicating that only 10% of women ages 18 to 26 initiated HPV vaccination in 2007.4 An emerging body of literature examining factors associated with HPV vaccine initiation and/or series completion has identified several significant predictors of uptake, including age,5⇓–7 race/ethnicity,5,7⇓⇓–10 student status,11 medical specialty,5,12 clinic type,7 insurance type,5,7⇓–9 urban status,6 neighborhood education level,9 historic health service utilization,6,9 receipt of meningococcal vaccine,6 use of contraception requiring intramuscular injections every 3 months,8 perceived personal importance of vaccination,11 and strength of physician's recommendation.11 Another critical barrier reported by parents is not receiving a recommendation for the HPV vaccine from a health care professional.13
The few published observational studies of adherence to dosing intervals used different definitions for “on-time” dosing. Tan et al7 examined factors associated with on-time dosing in a retrospective cohort study of female patients ages 9 to 26 with at least 1 HPV vaccine dose documented in the North Carolina Immunization Registry. During the 2-year study period, only 25% completed the HPV vaccine series on time, as defined by the dosing window used in the quadrivalent HPV vaccine trials, with significant differences in on-time series completion by age, race, ethnicity, insurance type, and clinic type. Widdice et al8 examined adherence to the dosing schedule recommended by the ACIP and factors associated with series completion within 7 and 12 months in a retrospective review of health records of 9- to 16-year-old patients who had initiated HPV vaccination at an academic medical center. They found low adherence to ACIP-recommended intervals; over half of doses were received late and only 28% of patients completed the 3-dose series by 1 year.
Reminder calls to families are, in general, effective for vaccine uptake.14 Only 3 studies that have examined reminder calls or prompts for adolescent vaccination have been published.15⇓–17 Only the recent study demonstrated a clinician-focused intervention that included electronic health record (EHR) alerts was most effective at initiating the HPV vaccination series.15 However, EHR alerts were part of a more resource-intense intervention, however, so the impact of turning on alerts cannot be determined.
The objectives of this study were to examine the effect of simply turning on an EHR alert for HPV vaccine initiation, series completion, and adherence to ACIP-recommended dosing intervals among eligible female patients. This less resource-intense approach will become more common in our practices. We hypothesized that the practice with EHR prompts for the HPV vaccine would have higher initiation, more timely completion of the series, and more patients completing the series.
Methods
Study Design
We used a retrospective cohort design.
Study Population
We defined 2 cohorts of females aged 9 to 26 years beginning in March 1, 2007, with at least 1 doctor appointment between March 1, 2007, and January 25, 2010, seen in 2 different community-based family medicine practices.
Study Setting
The exposure, or prompted, cohort comprised patients seen in 5 academic, community-based family medicine practices in the Midwest with a common institution-created EHR and electronic reminder system. The control, or unprompted, cohort comprised patients from another 4 academic, community-based family medicine practices in the Midwest with a common EHR without any electronic prompting or alert system for vaccines. The 2 academic centers do not have overlapping catchment areas. The available characteristics of the clinics from 2007 are summarized in Table 1 by prompted and unprompted cohort. There are a wide range of full-time equivalent faculty physicians, number of patient visits in 2007, and patients per full-time equivalent faculty physicians between clinics within a cohort and between cohorts, as highlighted in Table 1. Among the prompted cohort, 2 of 5 clinics have residents, whereas all clinics within the unprompted cohort have residents. All the clinics have medical students. The research ethics were reviewed by human research committees and approved by an institutional review board.
Outcomes
The primary outcome of interest was the initiation and completion of the HPV vaccine series, including time between each vaccine. The covariates of interest were age, race, and number of visits during the observation period.
Exposure and Intervention
The primary intervention was the exposure of the prompted cohort to HPV vaccine alerts during encounters with their health care providers. At the time of the patient encounter, providers (including physicians, nurse practitioners, physician assistants, and medical assistants) received the HPV vaccine prompt produced by the EHR. These practices had been using such a system since 2000, which included alerts for preventive and chronic care services.18 Vaccine alerts had been in place for a variety of childhood, adolescent, and adult vaccines. The HPV vaccine alert was started March 2, 2007. The provider alert was a simple list of services needed. For the HPV vaccine, the alert was “HPV” followed by the needed vaccine in the series. As part of this active prompting system, providers were required to respond to the HPV vaccine prompts with 1 of the following options: done, ordered, patient declined, patient not eligible, discussed, or not addressed. If results indicate not addressed or if additional vaccinations are due, the prompt returns at the following visit. Patients and parents received a brief note of “services your provider will recommend for you today.” Reminder algorithms were developed using Cielo Clinic (Cielo MedSolutions, Ann Arbor, MI) to prompt providers and patients at all appointments with females who are eligible to initiate or complete HPV vaccination at appropriate intervals.18
The unprompted cohort was seen in practices with an EHR without any systematic form of alerts for vaccines or other preventive services.
Analytic Variables
Patient Characteristics
Patient age was based on age at the start of the observation period and was categorized as 9 to 18 years or 19 to 26 years. Patient race was categorized as white, African American, or other. The total number of visits during the observation period was categorized as 1 to 2 visits or at least 3 visits with a clinician including a medical doctor (MD), doctor of osteopathic medicine (DO), nurse practitioner, or physician assistant. Visits made solely for vaccine delivery were not included in the analysis.
HPV Vaccine Initiation and Series Completion
Vaccine initiation was defined as receipt of at least 1 dose of the HPV vaccine during the observation period. Series completion was defined as receipt of all 3 doses of the HPV vaccine during the observation period. Variables were created to indicate opportunities to receive subsequent HPV vaccine doses, based on the lower limits recommended by the ACIP. Patients with at least 30 weeks elapsing after their first HPV vaccine dose were considered to have had the opportunity to complete the vaccine series during the observation period. We defined the time between doses using the date of vaccine from the EHR for each dose.
Statistical Analysis
Descriptive statistics on age, race, and number of vaccines received were calculated and compared between the 2 study cohorts using χ2 and independent samples t tests and between clinics within cohorts using χ2 and one-way analysis of variance. Multiple imputation was performed to impute values of race for subjects whose race was unknown. A multinomial logistic regression model, including covariates of age, study cohort, number of visits, and number of HPV vaccines received, was used to estimate race category probabilities for observations with missing race information. These probabilities were used to impute race information for a total of 10 imputed data sets. Analysis including race was performed on each imputed data set and the results were combined using the formula described by Little and Rubin.19
Correlates of vaccine initiation were examined using a clustered multivariable logistic regression model predicting receipt of ≥1 HPV vaccine dose versus no vaccination during the observation period. Correlates of vaccine series completion were examined in the subset of study patients who initiated the HPV vaccine series and had the opportunity to complete the series during the observation period, using a clustered multivariable logistic regression model predicting receipt of all 3 HPV vaccine doses versus receipt of any (ie, 1 or 2) doses during the observation period. Both models included the covariates of age, race, number of visits during the observation period, study cohort, and interactions of study cohort with all other covariates. Both models were fit using a generalized estimating equations approach with an exchangeable working correlation structure to account for practice clustering. Results are presented as adjusted odds ratios (ORs) and associated 95% confidence intervals.
Time to subsequent vaccine dose was estimated using the Kaplan-Meier product-limit estimator for time between doses 1 and 2 and doses 1 and 3 (completion) within the subset of subjects who initiated the series and for time between doses 2 and 3 within the subset of subjects who received a second dose during the study period. Differences in survivor functions by study cohort were tested using the log-rank test. Cox proportional hazard models were used to estimate the impact of study cohort on time to vaccination after adjusting for covariates for the same 3 intervals, using the same subsets of subjects as those used for the Kaplan-Meier estimates. Covariates in all 3 models included study cohort, patient age, patient race, and interactions between cohort and both race and age. Standard errors were calculated using a robust variance estimator method to account for practice clustering. Results are presented as hazard ratios with corresponding 95% confidence intervals. All analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Study Population
During the observation period, a total of 5994 females ages 9 to 26 in the prompted cohort and 9027 in the unprompted cohort were seen and eligible for the HPV vaccine. The prompted population had younger patients, on average (17.8 vs 18.5 years; P < .001). Race distribution was inconclusive given the ethnic race was not self-reported and was noted as “missing” in >33% of the unprompted study patients. Descriptive data of the study population are provided in Table 2.
The distribution of patients eligible for HPV vaccine by clinics within each cohort by age and vaccine delivery is summarized in Table 3. Within each cohort there were significant differences in age, race, and vaccine delivery between clinics. However, all the clinics within the prompted cohort had significantly higher rates compared with the clinics in the unprompted cohort.
HPV Vaccine Initiation
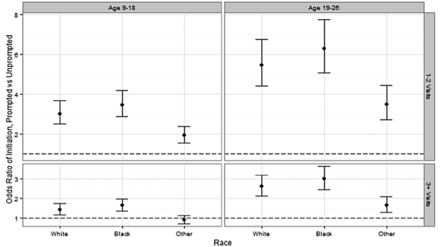
Overall, more vaccine-eligible females seen during the observation period initiated the series (received at least 1 dose) in the prompted cohort (35.0%) compared with the unprompted cohort (21.3%) (Table 1). Age, number of visits, and race were significantly associated with HPV vaccine uptake. Significant interactions between cohort and each covariate indicate differences in the cohort's effect on initiation for different levels of covariate values, which are illustrated in Figure 1. The prompted cohort had significantly higher odds of initiation at each level of covariates, with the exception of the subset of “other race” younger patients who had ≥3 visits with their primary care physician during the study period (OR, 0.9; P = .41). The highest effects were present in the older age group, who had fewer visits during the study period (ORs range from 3.5 to 6.3).
Odds ratios (95% confidence intervals) of series initiation at the prompted site versus the unprompted site, at the level of covariates. The odds ratios from clustered logistic regression predicting receipt of ≥1 human papillomavirus doses versus no vaccination during observation, including age, race, number of visits, and interaction of all variables with study site. Analysis also adjusted for practice clustering using generalized estimated equations with an exchangeable working correlation structure. The dotted line represents an odds ratio of 1, which would indicate no significant site effect.
HPV Vaccine Series Completion
A total of 1936 patients in the prompted cohort and 1706 in the unprompted cohort initiated and had the opportunity to complete the vaccine series. Age and race were significantly associated with series completion in the prompted cohort, whereas race and number of observations were significant in the unprompted cohort. The effects of study cohort on series completion are illustrated in Figure 2. The prompted cohort had significantly higher odds of completion when compared with the unprompted cohort for all levels of covariates; the highest effects were present for patients with fewer physician visits.
Odds ratios (95% confidence intervals) of series completion at the prompted site versus the unprompted site, at levels of covariates, among patients who initiated the human papillomavirus vaccine series and had the opportunity to complete the series during the observation period. The odds ratios from clustered logistic regression predicting receipt of all 3 vaccination doses versus any (i.e., 1 or 2) doses during observation, including age, race, number of visits, and interaction of all variables with study site. Analysis also adjusted for practice clustering using generalized estimated equations with an exchangeable working correlation structure. A total of 1936 patients at the prompted site and 1706 at the unprompted site had an opportunity to complete the series. The dotted line represents an odds ratio of 1, which would indicate no significant site effect.
Time to Subsequent Vaccine Doses
Patients in the prompted cohort were significantly more likely (P < .001) to receive all 3 doses on time and with shorter median intervals between each dose compared with those in the unprompted cohort, per the study-defined schedule. These differences are further highlighted in Figure 3.
Time to each vaccine dose. The shaded boxes represent the interquartile range (25th to 75th percentile); the solid vertical lines within the shaded boxes represent the median. Dots are outliers beyond 1.5 times the interquartile range from the 75th percentile. The red dashed lines in each panel represent the “on time” time range for each interval.
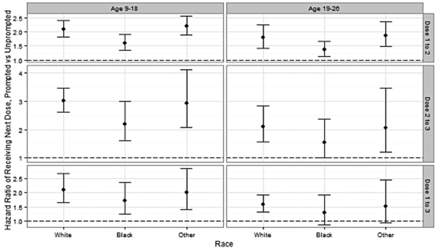
Results of Kaplan-Meier survival analyses and log rank tests indicated that survival functions for time to subsequent dose was significantly different between cohorts in all 3 time periods (doses 1 to 2, doses 2 to 3, and doses 1 to 3; P < .0001 for all). Hazard ratios between cohorts, estimated using Cox proportional hazard models (Figure 4), were significant in all situations with the exception of the comparison of prompted versus unprompted patients who were either African American or other race and those in the older age group with regard to the time between dose 1 and dose 3.
Time between dose 1 to 2, 2 to 3, and 1 to 3 by age and race for each cohort. Hazard ratios were estimated using Cox proportional hazard models, with clustered standard errors, including age, race, study site, age*site interaction, and race*site interaction. Analysis for doses 1 to 2 and doses 1 to 3 included the 4,019 patients who initiated the series. Analysis for doses 2 to 3 included the 2,731 patients who received a second dose during the study period. The dotted line represents a hazard ratio of 1, which would indicate no significant site effect.
Discussion
In this unique contrast of 2 community-based family medicine networks using retrospective cohorts, we demonstrated that clinics using an EHR with clinician and patient HPV prompts at office appointments resulted in significantly more young women initiating and completing the vaccine in a timely fashion. Whether using prompts or not, females age 9 to 18 years and those with ≥3 visits were significantly more likely to initiate the vaccine. The differences were more pronounced when contrasting the 2 clinical networks for these variables. African Americans in the prompted clinics were significantly more likely to initiate the HPV vaccine than whites. The prompts to both clinicians and patients may create a common agenda that facilitates the initiation of the vaccine and/or minimizes the unconscious discrimination of clinicians.20 Many reports have noted that African Americans are less likely to get the HPV vaccine.8,9,21 Clinicians could be unconsciously assuming that African Americans are not interested in the HPV vaccine.22 Therefore, the clinician does not bring it up given all the patient-centered demands competing during an appointment. In addition, discriminatory actions are more likely to occur when situational demands are unclear or when norms for appropriate actions are ambiguous.23 Given the time period of this study, women presenting for a primary care visit would not have a clear medical standard to be offered HPV vaccine. Systematic systems such as cues and alerts have been shown to reduce the difference between white and African American uptake of preventive services such as colorectal cancer screening.24 This study is the first to report an impact of alerts on vaccine uptake in young African American women.
We found that having ≥3 visits was associated with increased rates of initiation in both cohorts. This effect was, however, more pronounced among the unprompted cohort. There was no correlation with the number of visits to vaccination completion in the prompted cohort. Patients and providers in the prompted cohort seemed to be using the encounters to address the HPV vaccine in addition to the primary reason for the visit. As a result, fewer appointments were needed, which led to more efficiency. This highlights the promise of an electronic prompting system in improving vaccination uptake without increasing visits.
As hypothesized, the timeliness of vaccination across all 3 doses of the HPV vaccine series also was demonstrated to be superior among the prompted cohort compared with the unprompted cohort. The prompts may reflect completion during subsequent visits for reasons other than vaccination only. Or, the clinics with the prompts may have other systems in place to ensure appointments just to complete the vaccine are scheduled. We are not aware of any systematic protocols in any of the 5 clinics using the prompts during the observation time period. We did not count appointments with a nurse or medical assistant for vaccination only. The visits counted were with a clinician.
There are limitations to this study. This was not a randomized controlled trial allocating clinics to HPV vaccine prompt and no prompts. This was a retrospective cohort study of 2 different clinical networks during the same time period. Factors other than the EHR vaccine alert for HPV may account for the noted differences in initiation, completion, and interval between vaccines. These other factors could be community or population acceptance of the vaccine, insurance coverage, access to health care, competing health issues, clinician attitude about the vaccine, and office organization. We do not have the data to examine these and other variables. The prompted clinics also were seeing alerts for other childhood, teen, and adult vaccines. The HPV vaccine was not the only vaccine prompt; therefore, the unique focus of clinics and clinicians on the HPV vaccine in the alerts was unlikely. We focused only on female patients given that the vaccine was approved only for women during the study period. We are not able to comment on the impact of male patients' uptake and completion of HPV vaccine. Finally, the observation period was early in the rollout of the HPV vaccine. Other environmental and cognitive factors that have been linked to preventive uptake and completion of preventive services may be different now.24 Therefore, uptake and completion of HPV vaccines may also be different now. However, our initiation and completion rates were higher than a recently published trial of an intervention done in 2010 to 2011.15 Given the recent data on an alternative number of shots and schedules for HPV vaccine, striving for completion of the 3-dose HPV vaccine series at these intervals may not be critical.25⇓–27
Conclusion
This study demonstrated that simply alerting patients and clinicians during an office appointment with females aged 9 to 26 years increases the uptake and completion of the HPV vaccine series. Another study of prompts for HPV vaccine was not just an intervention of EHR-generated alerts.15 The intervention included (1) HPV vaccine alerts, (2) a 1-hour presentation about the alerts and review of practice-based HPV vaccine rates, and (3) quarterly performance feedback about HPV vaccine rates. This intervention increased vaccination rates by 9, 8, and 13 percentage points for each HPV dose and accelerated vaccination by 151, 68, and 93 days, respectively.15 This was a much more resource-intense intervention compared with simply turning on a new vaccine alert. The prompted clinics had a 15-percentage point increase in HPV vaccine completion. The vaccine dosing was accelerated, on average, by 60, 30, and 70 days between doses 1 and 2, doses 2 and 3, and doses 1 and 3 compared with the unprompted clinics. Our less resource-intense intervention had similar improvements. All EHRs should have a functionality for vaccine alerts as a core component. Further refinements of this process need to be examined to push higher the uptake and timely completion of HPV vaccine. We also need to examine outreach that moves beyond the patient–clinician encounter and may have an even greater impact.
Notes
This article was externally peer reviewed.
Funding: Financial support was provided by a grant from the National Cancer Institute (CA80846) and the Dr. Max and Buena Lichter Research Professorship in Family Medicine (MTR).
Conflict of interest: MTR is conducting a study funded by the National Cancer Institute, and Merck provides free human papillomavirus vaccine to the study participants and pays for serum assays. Merck had no role in the design, implementation, or analysis of this study.
- Received for publication March 6, 2014.
- Revision received November 21, 2014.
- Accepted for publication December 1, 2014.