Dressler syndrome is a well-known complication of myocardial infarction and postsurgical instrumentation of the pericardium (as postpericardiotomy syndrome). Also called postmyocardial infarction syndrome, it typically occurs 3 weeks to several months after the surgery, with symptoms of fatigue, malaise, fever, chest pain, pleurisy, pneumonia, and left shoulder pain.1 Diagnostic evaluation may reveal an elevated sedimentation rate, leukocytosis, eosinophilia, pericarditis, and pleural effusions.2 Minimally invasive coronary artery bypass graft (CABG) surgeries avoid full midline sternotomy, aortic cannulation/manipulation, and use of cardiopulmonary bypass and achieve the same beneficial effect as traditional bypass surgeries with lower rates of complications.3 Although an English-language literature review from 1996 to 2003 revealed no reports of Dressler syndrome after minimally invasive CABG, the following case indicates that Dressler syndrome can occur after this procedure, probably because the pericardium is breached.
Case Reports
A 78-year-old woman presented to the family practice office complaining of fatigue, malaise, and dyspnea since her minimally invasive CABG surgery 1 month earlier. Three days before admission, she also developed a dry cough and pleuritic chest pain. One day before admission, she noted a fever. She denied exertional chest pain, hemoptysis, pedal edema, or calf pain. On physical examination, her temperature was 100.4°F, pulse was 106 beats/min, respiration was 20 breaths/min, and pulse oximetry measured 98% blood oxygen saturation on room air. Pulmonary examination revealed dullness to percussion with decreased breath sounds and decreased tactile fremitus at the left base. No murmur or rub was noted on cardiac examination. There was no pedal edema or calf tenderness.
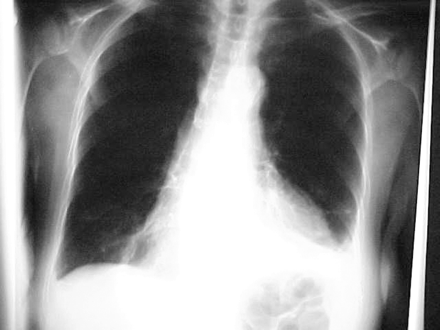
Results of laboratory tests were notable for a white blood cell count of 12,900, hemoglobin of 10.7 g/dL, and platelet count of 694,000. Cardiac enzymes were within normal limits. Chest radiograph showed left greater than right pleural effusions with right lower lobe atelectasis (Figure 1). A left lower lobe consolidation could not be excluded. Electrocardiogram showed no significant changes.
Chest radiograph taken on patient admission.
The patient was admitted to the hospital for presumed community-acquired pneumonia and started on azithromycin and ceftriaxone. Despite 3 days of antibiotic therapy, the patient continued to have fevers (100.1°F to 100.2°F) each afternoon and reported no subjective improvement. Blood cultures and electrocardiogram were normal. The platelet count remained high at 897,000, and erythrocyte sedimentation rate (ESR) was noted to be 122 mm/hour. A repeat chest radiograph was essentially unchanged. Decubitus films and an ultrasound revealed a small left-sided pleural effusion that was not amenable to thoracentesis. A definite area of consolidation could not be identified on the decubitus films. Echocardiogram was negative for a pericardial effusion.
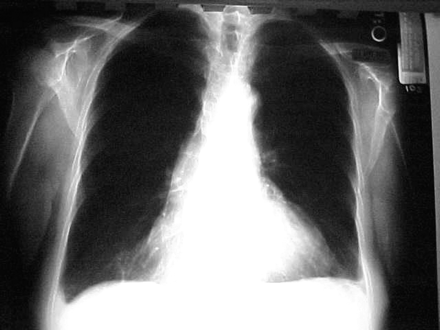
A diagnosis of Dressler syndrome was considered, and treatment was initiated with indomethacin. The patient’s temperature subsequently normalized within 24 hours. The patient began to feel some subjective improvement, although her platelet count remained high at 990,000 and the ESR remained elevated at 142 mm/hour. Indomethacin was discontinued and 20 mg of prednisone was given twice daily. The patient was discharged home on hospital day 8. At follow-up 2 weeks later, she was subjectively improved while taking the steroids, and the ESR and platelet count had decreased to 53 mm/hour and 330,000, respectively. Her hemoglobin had increased to 12.1 g/dL. Follow-up chest radiograph (Figure 2) showed almost complete resolution of the pleural effusion and no evidence of a consolidation.
Chest radiograph taken after 2 weeks of steroid therapy.
Discussion
Dressler syndrome has an incidence of approximately 5% after cardiac events, with 50% being diagnosed in the second or third week after infarction.4 The syndrome may have a higher incidence (up to 30%) after surgical procedures and is typically characterized by low-grade fever, malaise, and left-sided pleuritic pain.2,5 Leukocytosis, an elevated sedimentation rate, eosinophilia, and anti-heart antibodies are expected.4 Chest radiograph will identify pleural effusions in up to 60% of the cases, and these effusions may require thoracentesis for characterization and symptomatic relief.4 Up to 75% of patients may have a friction rub, and most will have a pericardial effusion on echocardiography.4
The differential diagnosis should include congestive heart failure, pulmonary embolism, pneumonia, myocardial infarction, and possible malignancy.5 The clinical appearance of the patient should guide the workup, which may include thoracentesis, ventilation-perfusion scan, blood cultures and cardiac enzymes as indicated.
The cause of Dressler syndrome is unknown, although an autoimmune link has been proposed.2 Antimyocardial antibodies are often observed serologically but are of little clinical significance.4 It is unclear whether the anti-heart antibodies precipitate or result from the syndrome.5 In a report of patients undergoing surgical procedures involving the pericardium, all those who had high titers of anti-heart antibodies subsequently developed Dressler syndrome. Those without the rise in titers failed to develop the syndrome.5 An early pericardial reaction may predispose a patient to Dressler syndrome and may represent a delayed autoimmune or inflammatory response to a focal pericarditis.4
Treatment for Dressler syndrome is aspirin or nonsteroidal anti-inflammatory drugs to decrease fever and pain.4,6 Corticosteroids may be used for persistent symptoms.4,6 Recurrence is common, and relapses have been reported up to 1 year after the initial event.4,6 Serious complications include cardiac tamponade, constrictive pericarditis, and occlusion of the bypass graft.4
Although not a common disorder, Dressler syndrome should be considered in those patients who have persistent fatigue and malaise after a myocardial infarction or CABG procedure, especially with onset greater than 2 weeks after the event. Our patient’s presentation, lack of other identifiable cause, and prompt response to treatment suggested Dressler syndrome. We could find no other reported cases of Dressler syndrome after minimally invasive CABG in the English literature.
Minimally invasive CABG, which does not require sternotomy or cardiac standstill, was developed as an alternative to standard CABG. Because the integrity of the pericardium is breached during minimally invasive CABG, albeit to a lesser degree than with standard CABG, Dressler syndrome may be an expected sequela. This case indicates that Dressler syndrome may follow minimally invasive CABG.
Notes
The opinions presented in this article represent those of the authors and do not represent the opinions of the United States Navy.
- Received for publication July 16, 2003.
- Revision received July 16, 2003.