Abstract
To date, user-friendly, practical guidelines for dementia have not been available for busy family physicians. However, the growing number of patients with dementia means that primary care physicians will have an increasingly important role in the diagnosis and subsequent management of dementia. This article provides practical guidance for the recognition and diagnosis of dementia and is aimed at family physicians, who are usually the first clinicians to whom patients present with dementia symptoms. Because Alzheimer disease (AD) is the most common form of dementia, this condition is the main focus of this article. We review the pathophysiology of AD and discuss recommended diagnostic protocols and the importance of early diagnosis. An AD diagnostic algorithm is provided, with clearly defined steps for screening and diagnosing AD and assessing daily functioning, behavioral symptoms, and caregiver status.
Dementia: an Overview
Alzheimer disease (AD) is the most common form of dementia, accounting for approximately 60% of all cases,1,2 and it is therefore the main focus of this article. AD destroys brain cells, causing problems with memory, thinking, and behavior severe enough to affect work, family, and social relationships and, eventually, the most basic activities of daily living (ADLs). AD and related disorders are a growing public health problem in the United States, with a prevalence ranging from 3% to 11% among people aged 65 years and older and from 25% to 47% among those aged older than 85 years.3,4 There are an estimated 5.3 million cases of dementia in the United States,5 and this number is expected to increase to 18.5 million by 2050.6 The number of those afflicted is increasing annually as a result of the aging population. Dementia leads to a high burden of suffering for patients, families, and society, with an annual estimated cost of $172 billion.5
People with dementia usually present first to their family physician, although an estimated 39% present to specialist clinics (neurologists, psychiatrists and geriatricians).7 The primary care physician (PCP) is often the first physician to observe patients with possible dementia and often the only physician involved in making the diagnosis.8 Because of the key role that PCPs play in the long-term management of elderly individuals with chronic disease, the growing number of patients with dementia will have a significant impact on these health professionals.9
The rising number of patients with dementia means that family physicians will have an increasingly important role in recognizing early signs and symptoms of disease, ordering appropriate tests, formally diagnosing, and, finally, treating these patients. However, at present, diagnosing AD can be challenging. In the early stages, AD can be difficult to distinguish from the decline in cognitive abilities due to normal aging and the mild cognitive impairment (MCI) that often precedes AD.10,11 Furthermore, there are insufficient numbers of neurologists to care for all the patients with MCI and dementia, and there are a limited number of dementia specialists available for consultation. It is, therefore, imperative that PCPs learn how to assess their patients for dementia; specialty clinics cannot deal with the numbers of patients—a problem that will only worsen as the population continues to age.
To date, user-friendly, practical guidelines have not been available for busy family physicians. Indeed, studies suggest that family physicians may have a relatively limited knowledge of dementia.12,13 Thus, there is a pressing need to facilitate diagnosis, which can be simplified by adherence to clearly defined guidelines. This article provides practical guidance for the recognition and diagnosis of dementia and is aimed at PCPs, who are usually the first clinicians to whom patients present with dementia symptoms.
Pathophysiology of AD
AD is a progressive neurodegenerative disorder that represents the most common form of dementia. The most prominent clinical feature of AD is an early impairment of episodic memory,2 which manifests as memory impairment of recent events, unusual repeated omissions, and difficulty learning new information. For example, an individual with AD may ask the same question repeatedly throughout the day, such as what they are going to eat for dinner, or frequently misplace items (glasses, keys, or wallet). The loss of memory, especially for learning and retaining new information, reflects impaired function in the hippocampus and other medial temporal lobe structures, which are sites of early pathologic change.2 As the disease progresses, the symptoms often manifest in more persistent language disturbance and difficulties completing more complex tasks of daily living. Patients progress from loss of higher level ADLs, such as the ability to perform financial transactions and drive a car or use public transportation, to abnormalities in the more basic ADLs (eg, personal hygiene, toileting). Behavioral problems frequently develop and include depression, apathy, anxiety, agitation, psychosis (delusions and hallucinations), wandering, and aggression.2
Guidelines for Diagnosis
The diagnosis of AD is usually based on the National Institute of Neurological, Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA).14 According to these guidelines, the diagnosis is classified as definite (clinical diagnosis with histologic confirmation), probable (typical clinical syndrome without histologic confirmation), or possible (atypical clinical features but no alternative diagnosis apparent; no histologic confirmation). The currently accepted criteria support a probabilistic diagnosis of AD within a clinical context where there is no definitive diagnostic biomarker. A definite diagnosis of AD is only made according to the NINCDS-ADRDA criteria when there is histopathologic confirmation of the clinical diagnosis.15 Autopsy results support the “probable” clinical diagnosis in 86% to 90% of cases.16
Although the NINCDS-ADRDA criteria for AD are the prevailing diagnostic standards in research, these have now fallen behind the unprecedented growth in the understanding of the disease process.15 The clinical phenotype of AD is no longer described in exclusionary terms, but it can be characterized more definitively on a phenotypic basis. Distinctive markers of the disease are now recognized, including structural brain changes visible on magnetic resonance imaging with early and extensive involvement of the medial temporal lobe, molecular neuroimaging changes seen with positron emission tomography (PET), and changes in cerebrospinal fluid (CSF) biomarkers.15 There recently has been intense research interest in characterizing the earliest stages of disease that predate the crossing of the dementia threshold, defined by functional disability. Prodromal AD is the symptomatic predementia phase of AD, generally included in the MCI category; this phase is characterized by symptoms not severe enough to meet currently accepted diagnostic criteria for AD.15 The presence of at least one biologic footprint of AD should improve the specificity for diagnosis. This concept forms the foundation of the new diagnostic criteria proposed in 2007, which were developed to allow an earlier and more specific AD diagnosis.15
The proposed criteria move away from the traditional 2-step approach of first identifying dementia according to degree of functional disability and then specifying its cause.15 Instead, they aim to define the clinical, biochemical, structural, and metabolic presence of AD at the earliest stages before full-blown dementia. These new criteria are centered on a clinical core of early and significant episodic memory impairment. They stipulate that there also must be at least one or more abnormal biomarkers among structural neuroimaging with magnetic resonance imaging, molecular neuroimaging with PET, and CSF analysis of β-amyloid or τ proteins.15 For example, a decrease in the β-amyloid (1–42) peptide and an increase in the τ and phospho-τ proteins may be the earliest signs of AD.2,10,17 These criteria represent a cultural shift requiring a more biologically focused workup than previous approaches, and their timeliness is highlighted by the development of drugs that are directed at altering pathogenesis, particularly at the production and clearance of β-amyloid.2,15
Although the focus of research recently has shifted to the development of new tools that increase the specificity of the prodromal AD diagnosis,2 there are no readily available biomarkers, laboratory tests, or brain imaging techniques for routine use to aid diagnosis.18,19 As such, their use has limited application for family physicians at this time. Likewise, there are no genetic markers currently recommended for routine use in the diagnosis of AD, including testing for the apolipoprotein E4 gene or for mutations in amyloid, presenilin, or τ that have been linked to rare forms of familial dementia.18 It therefore remains necessary to screen patients by taking a diligent approach to assessing cognition and function.
Importance and Challenges of Early Diagnosis
Making a diagnosis of dementia in the early stages can be a clinical challenge. The insidious and variable emergence of dementia symptoms makes recognition of the syndrome problematic, particularly in the primary care setting,20 with the often limited time available for consultation. In addition, physicians need to be wary of patients' ability to hide their symptoms. In the early stages of dementia, accommodation to or denial of changes in cognition, functional ability, mood, or behavior are common coping strategies.21 As the person's denial strengthens, the concerns of the family become more pressing, with the physician often caught in between and faced with apparently irreconcilable needs.21 Most specialty associations, including the American Academy of Neurology, the American Geriatrics Society, the American Medical Association, and the American Association of Family Practitioners, encourage physicians to be alert to cognitive problems in older adults and to take steps to identify cognitive disorders at the earliest possible stage. However, clinicians in primary care often fail to screen older adults for AD on a routine basis because of insufficient time, inadequate reimbursement for services, and uncertainty about resources available to facilitate diagnosis.22,23 Furthermore, given the wide range of services needed, PCPs often fail to make referrals because of insufficient knowledge about resources.23,24
Although it is recognized that current pharmacologic and behavioral interventions do not prevent eventual disease progression, there is good evidence to show that their use can lead to disease stabilization and delayed progression of cognitive, functional, and behavioral outcomes, and these outcomes may provide improvement in quality of life for the patient and their family.22 Nevertheless, family physicians should counsel patients with AD and their families about realistic expectations of treatment outcomes with these agents, which are likely to be modest,25 and the potential for mild to moderate adverse effects (mostly nausea, vomiting, diarrhea, or all three). Because damage caused by the pathophysiologic mechanisms associated with AD is presumed to be irreversible, early detection of AD offers better prospects for patients with AD and their families and friends. This allows both the patient and family to participate in their care plan and to prepare better for future challenges as a result of the neurodegenerative process26,27 because currently available medications, and any future disease-modifying agents, will have the greatest opportunity for providing benefit. In addition, recent evidence suggests that early treatment provides economic benefits both to the patient and caregiver as well as society as a whole.28⇓–30 Hence, the early establishment of a diagnosis and subsequent initiation of an appropriate management program can optimize the prognosis for patients with AD.
Despite the benefits of early intervention, dementia remains underdiagnosed; an estimated 50% of primary care patients aged older than 65 years have not been diagnosed by their PCPs. A primary reason cited for the delay in AD diagnosis has been the difficulty in identifying early signs of AD by both PCPs and the general public.31 Given the challenges and benefits of timely and accurate diagnosis of neurodegenerative disorders, physicians and patients alike desire tools that aid in dementia diagnosis as early as possible.32
AD Diagnosis Algorithm
Diagnosis of dementia is a stepwise process that involves examination of patient history and early warning signs, as well as performance screening, assessment of daily functioning, behavioral problems, and caregiver status, with possible referral to specialist clinics for more thorough assessment (Table 1).
Step 1: Prediagnostic Tests and Early Warning Signs
Before any diagnostic tests are performed, patient history, physical examination, and laboratory findings as well as input from family should be considered because these factors are paramount to the diagnosis of dementia (Table 1).33 In particular, family physicians should take into account any risk factors for AD that may be present, including older age, lower education, female sex, and family history of AD (Table 1).
There are several conditions that mimic dementia (eg, depression, hypothyroidism) that can be missed if not evaluated and, if detected, are potentially reversible. To diagnose AD, other medical, neurologic, or psychiatric disorders that could account for the impairment in memory and related symptoms must be investigated1 (Table 1). Furthermore, family physicians need to be aware of the potential for vision and hearing problems that could be contributing in a significant manner to an apparent decline in cognitive function. It also should be noted that patients with AD frequently have comorbid medical conditions (Table 1), the presence of which can further impair patient function; their appropriate treatment can optimize outcomes and minimize excess disability.12 Laboratory tests are necessary to identify or rule out secondary causes of dementia and coexisting disorders that are common among elderly people.1 Recommended tests are shown in Table 1.
Even before the onset of cognitive problems, there are several warning signs that can predict those individuals at a higher risk of developing dementia, including increased frequency of patient visits to their family physician before diagnosis.34,35 There are also somatic changes that precede the onset of dementia that may provide important clues, including accelerated weight loss,36 gait disturbances, physical frailty,37 and slowed psychomotor speed36 (Table 1).
Ten Key Warning Signs for AD
The Alzheimer's Association lists 10 key warning signs of AD10:
Memory loss
Difficulty performing familiar tasks
Problems with language
Disorientation to time and place
Poor or decreased judgment
Problems with abstract thought
Misplacing things
Changes in mood or behavior
Changes in personality
Loss of initiative
In many patients, the most clinically prominent feature of AD is the decline in cognitive function with an early impairment of episodic memory, for example, what the patient cannot recall what they had for breakfast, even with the provision of cues.1 Memory complaints are thus the most reliable and easiest to test for signs of early AD.
Step 2: Screening Tools Employed by Family Physicians
It is crucial that physicians screen for AD under appropriate conditions with validated screening tools that are suitable for a busy practice. Several screening tools are available for use in primary care and are continually being updated and re-evaluated to provide family physicians with brief, easy to administer, and effective diagnostic tools. All screening tools are readily available to family physicians and can be obtained without incurring costs. Many family physicians may find it helpful to select a few screening tools and become familiar and proficient with them. It should be noted, however, that these screening tools are not specifically diagnostic for AD. Rather, they provide evidence that an impairment exists and a rationale to move to a more formal evaluation for confirmation.
There are 2 basic ways to assess the patient for dementia. One approach is a performance measure in which a test is administered and scored. The score then is compared with a published norm. This method is useful for determining how the patient performs in comparison with other age-matched individuals, and scores can be followed over time; however, unless previous baseline testing was performed, the score is not able to give the clinician information about whether the patient's cognitive abilities have changed, nor whether the impairment interferes with everyday activities. In addition, brief performance measures may be biased by age, race, sex, education, and socioeconomic status. A second approach is an informant interview. An observant informant can provide information about how the patient's cognitive abilities have changed and whether the change interferes with everyday activities. Because each patient serves as his or her own control, these assessments suffer less from biases. However, the limitation may be in finding an informant for older adults who live by themselves or in skilled nursing facilities. A combination approach with an informant and performance measure may improve the ability to detect dementia at the earliest stages.38
Performance-Based Screening Tools
In terms of assessing cognitive function, the Mini-Mental State Examination (MMSE) test has been used frequently for initial assessment of AD, and its sensitivity increases if a decline of the score over time is taken into account7,17 (Table 2). Although the MMSE is quick and easy to administer and can track the overall progression of cognitive decline, it is not considered to be a good test for definitive AD diagnosis.1 In addition, there are several issues associated with the MMSE, including bias according to age, race, education, and socioeconomic status (Table 2).39
Several screening tools are now available for use in primary care as alternatives to the MMSE7,17; these are being updated continually to provide PCPs with brief, easy to administer, and effective diagnostic tools. The Mini-Cognitive Assessment Instrument (Mini-Cog) has sensitivity and specificity for dementia similar to that of the MMSE (Table 2).40 However, the Mini-Cog's brevity may be an advantage when trying to improve recognition of cognitive impairment in primary care.40,41 In addition, the Mini-Cog is not associated with the same language or education bias as the MMSE.39,42 An example of the Mini-Cog test is provided in Appendix 1.
Newer instruments, such as the Montreal Cognitive Assessment, a screening tool developed to assist PCPs in detecting MCI, are gaining credibility because of improvements in sensitivity and decreasing susceptibility to cultural and educational biases (Table 2).43 Although more complex than the MMSE and Mini-Cog, the Montreal Cognitive Assessment offers the advantage of testing multiple cognitive domains with an easy scoring system and is free for clinical use (www.mocatest.org). There are many other short and simple memory tests available that can be used as first-line screening tools for use in primary care.17 Each has its pros and cons; the important point is that an objective measurement can provide an accurate “snapshot” of the patient's cognitive abilities and permits a quantitative measurement to follow for evidence of a treatment response.
Informant-Based Screening Tools
Another key test used in primary care is the 8-item Ascertain Dementia (AD8) screening interview, which is a brief, sensitive measure that reliably differentiates between individuals with and without dementia by querying memory, orientation, judgment, and function (Table 2). The AD8 can be completed by the informant in the waiting room before the office visit. In the absence of an informant, the AD8 also can be completed by the patient as a self-rating tool.44 Because the diagnosis of dementia requires a cognitive deficit that represents a change from premorbid abilities and interferes with social and occupational functioning, an informant assessment provides information that a performance measure cannot. Informant interviews such as the AD8 may be more sensitive to early stages of dementia and have strong correlation with biological markers of AD such as CSF and PET studies.45 An example of the AD8 test is provided in Appendix 2.
Of the tests described above, the Mini-Cog and AD8 are recommended as key diagnostic tools to use in primary care because they are brief, valid, and reliable instruments that are easy to administer, clinically acceptable, and effective. Both have psychometric properties superior to the MMSE; as such, they are clinically and psychometrically robust and, it has been argued, are more appropriate for routine use in primary care.27,46
Step 3: Assessment of Daily Functioning
An assessment of daily function is vital to determine the extent of the patient's disability and dependence on the caregiver, the results of which help to enable planning to maximize patients' independence.47 Basic ADLs, such as feeding and toileting, can be assessed with an interview or by using a tool such as the ADL Scale. Assessment of instrumental ADLs (IADLs) addresses more advanced activities, such as shopping, cooking, and managing finances. The IADL scale is used most frequently and measures 7 areas of more complex activities required for optimal independent functioning48,49 (Table 2; Appendix 3). The cognitive changes commonly associated with AD first impact the instrumental and, eventually, the basic ADLs.50⇓–52 Another commonly used scale is the Functional Assessment Questionnaire (FAQ),53 which includes 10 items and has been developed from the IADL scale. It assesses shopping, handling finances, preparing a meal, and traveling (which are also in the IADL scale); remembering appointments; and paying attention to, understanding, and discussing television, a book, or a magazine. The total score ranges from 0 (independent) to 30 (dependent).53 An example of the FAQ is provided in Appendix 4.
Step 4: Assessment of Behavioral Symptoms, Psychotic Symptoms, and Depression
More than 80% of patients with AD experience some form of behavioral symptoms such as anxiety, agitation, depression, and apathy during the course of the disease,54⇓–56 and patients should, therefore, be assessed periodically. Although these symptoms may be observed by the family physician, they are more often reported by the primary caregiver.13
Standardized tools can be used by PCPs or clinic staff to gather information about behavioral symptoms from the caregiver and evaluate effectiveness of interventions over time. The Neuropsychiatric Inventory Questionnaire is a quickly administered instrument that provides reliable assessment of behaviors commonly observed in patients with dementia57 (Table 2; Appendix 5). In addition, the Behavioral Pathology in Alzheimer's Disease (BEHAVE-AD) Rating Scale was designed particularly to be useful in prospective studies of behavioral symptoms and in pharmacologic trials to document behavioral symptoms in patients with AD.58 The BEHAVE-AD Rating Scale has 2 parts: the first concentrates on symptomatology and the second requires a global rating of the symptoms on a 4-point scale of severity. The domains covered are paranoid and delusional ideation, hallucinations, activity disturbances, aggression, diurnal variation, mood and anxieties, and phobias.58
Step 5: Caregiver Needs and Status/Support system
Family caregivers are central to the PCP's assessment and care of the patient with AD,59 and establishing and maintaining collaboration with caregivers is critical for care of the AD patient.59,60 Indeed, major physician organizations, such as the American Academy of Neurology61 and the American Association for Geriatric Psychiatry,62 have emphasized the importance of family caregivers by encouraging family physicians to form partnerships with families who care for dementia patients (Table 1).
In addition, the physical and emotional health of the primary caregiver is crucial in obtaining optimal care for the AD patient. Caregivers suffer from increased rates of depression and physical illness,13 and family physicians need to monitor regularly the health of the primary caregiver as well as that of the patient with AD (Table 1). Assessing caregiver status can lead to the implementation of measures that minimize patient–caregiver stress and delay institutionalization of the patient.
Additional Considerations
The family physician should be aware of the need for cultural, language, and literacy assessment within the clinical evaluation process for AD. Identifying the patient's and family's culture, values, primary language, literacy level, and decision-making processes will enable optimal assessment and management of AD patients and their families (Table 1).
Referral: Role of the Specialist and Further Investigations
Difficulty making a diagnosis may necessitate referral to a specialist, such as a geriatrician, neurologist, or psychiatrist, or require the request for further investigations to be completed by a neuropsychologist. For those with mild or questionable impairment, more comprehensive standardized cognitive assessments can be useful to assist with establishing a firm diagnosis; these are normally undertaken as part of a specialist assessment after referral.7
Conclusion
The growing number of patients with dementia means that PCPs will have an increasingly important role in the diagnosis and subsequent management of disease. There has been unprecedented growth of scientific knowledge about AD and a subsequent move toward its earlier diagnosis. However, in the absence of biomarkers and brain imaging that can be used routinely in primary care, the emphasis remains on the family physician's ability to recognize and diagnose AD using performance screening tools. Of the newly available screening tools for use by PCPs, the Mini-Cog and AD8 are particularly useful as complementary, brief, easy to administer, and effective diagnostic assessments that can be used in everyday clinical practice. Alongside cognitive and daily functioning assessments, a thorough evaluation of behavioral symptoms and caregiver status is required to ensure that both the patient and the patient's family receive optimal care.
Acknowledgments
The authors would like to thank Frances Gambling for her editorial assistance with the manuscript. Administrative, editorial, and technical assistance was funded by Novartis Pharmaceuticals Corporation.
Appendix 1
The Mini-Cog Test
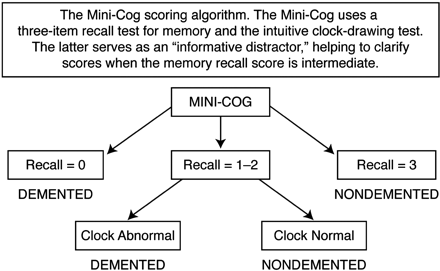
Scoring: 1 point for each recalled word (Figure 1).
The Mini-Cog Scoring Algorithm. 0 = Positive for cognitive impairment; 1 or 2 plus an abnormal clock drawing test (CDT) = positive for cognitive impairment; 1 or 2 plus a normal CDT = negative for cognitive impairment; 3 = Negative screen for dementia (no need to score CDT). Reproduced with permission from Borson S et al. Int J Geriatr Psychiatry 2000;15:1021–1027. © 2000 John Wiley & Sons39
Score the clock drawing test (CDT) as normal (the patient places the correct time and the clock appears grossly normal) or abnormal.
CDT Instructions
A pen/pencil and a blank sheet of paper are required.
Instruct the patient to listen carefully and repeat the following:
Apple
Watch
Penny
Administer the CDT: ask the patient to draw a traditional clock face showing a time that is potentially confusing for someone with AD, such as “10 minutes past 11.”
Ask the patient to repeat the 3 words given previously.
Scoring (number of correct items recalled):
3 = Negative screen
1 or 2 plus normal CDT = Negative screen
1 or 2 plus abnormal CDT = Positive for cognitive impairment
Appendix 2
The AD8 Administration and Scoring Guidelines
A spontaneous self-correction is allowed for all responses without counting as an error.
The questions are given to the respondent on a clipboard for self-administration or can be read aloud to the respondent either in person or over the phone (Table 3). It is preferable to administer the AD8 to an informant, if available. If an informant is not available, the AD8 may be administered to the patient.
When administered to an informant, specifically ask the respondent to rate change in the patient
When administered to the patient, specifically ask the patient to rate changes in his or her ability for each of the items without attributing causality
If read aloud to the respondent, it is important for the clinician to read the phrase carefully as worded and give emphasis to note changes caused by cognitive problems (not physical problems). There should be a 1-second delay between individual items.
No time frame for change is required
The final score is a sum of the number items marked “yes, a change”
Interpretation of the AD863
A screening test in itself is insufficient to diagnose a dementing disorder. The AD8 is, however, quite sensitive to detecting early cognitive changes associated with many common dementing illnesses including AD, vascular dementia, Lewy body dementia, and frontotemporal dementia.
Scores in the impaired range (see below) indicate a need for further assessment.
Scores in the “normal” range suggest that a dementing disorder is unlikely, but an early disease process cannot be ruled out.
More advanced assessment may be warranted in cases where other objective evidence of impairment exists.
The following cut points are used to diagnose dementia:
0 or 1: Normal cognition
≥2: Cognitive impairment is likely to be present
Appendix 3
Instrumental Activities of Daily Living13
This tool evaluates the patient's ability to perform the more complex activities that are necessary for optimal independent functioning (Table 4). The scoring indicates whether the patient is completely independent (I), requires assistance (A), or is dependent (D) for the performance of each activity. The IADL instrument may be repeated periodically to determine the need for more support.
Appendix 4
Appendix 5
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: Dr Sadowsky has served as a consultant to Novartis Pharmaceuticals Corporation, has served as a speaker for Novartis Pharmaceuticals Corporation and Forest Pharmaceuticals and Accera, and has received honoraria from these companies. James E Galvin is a paid consultant for Pfizer, Eisai, Novartis Pharmaceuticals Corporation, Ortho-McNeil and Forest. Neither Dr Sadowsky nor Dr Galvin received financial support for this manuscript.
- Received for publication August 4, 2010.
- Revision received October 17, 2011.
- Accepted for publication October 19, 2011.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.
- 66.
- 67.
- 68.
- 69.
- 70.
- 71.