Abstract
Single maintenance and reliever therapy (SMART) is an asthma treatment approach that utilizes combined inhaled corticosteroids and long-acting β-agonists for maintenance and quick relief therapy. Despite the evidence for its benefits in asthma treatment and its adoption into American and international asthma guidelines and recommendations, SMART remains a practice of some debate. This article reviews the available evidence for SMART and offers guidance for its integration into comprehensive asthma management. Overall, short-acting β-agonist-only asthma therapy regimens should be avoided, regardless of condition severity (SOR A Recommendation). Family medicine clinicians should start SMART for patients requiring either GINA Step 3 or 4 therapy, especially if they have signs of poor adherence (SOR B Recommendation). Finally, use budesonide-formoterol over other inhaled corticosteroid/long-acting β-agonist combinations when implementing SMART (SOR B Recommendation).
- Anti-Asthmatic Agents
- Asthma
- Evidence-Based Medicine
- Pharmacotherapy
- Primary Health Care
- Single Maintenance and Reliever Therapy (SMART)
For decades, the mainstay of asthma treatment has been inhaled β-agonists and inhaled corticosteroids (ICS). Traditionally used as rescue and maintenance therapies respectively, recent data question this approach as evidence-based. β-agonists like albuterol act on adrenergic receptors, activating adenylyl cyclase and preventing airway smooth muscle contraction, but do not reduce underlying causative inflammation. ICS prevent asthma exacerbation on a pathophysiologic level by reversing capillary permeability, reducing inflammation, and reducing the risk of bronchial hypersensitivity, but offer limited acute relief. Despite these benefits, patient ICS adherence is poor, increasing the risk for exacerbation and asthma progression.9 Thus, the idea was born to use combination ICS/β-agonist formulations to improve ICS exposure among patients with asthma.
Single maintenance and reliever therapy (SMART) is an asthma treatment approach using an ICS and long-acting β-agonist (LABA) for maintenance and quick relief therapy as a single inhaler.10 Through this approach, patients continue usual step-directed maintenance therapy and use additional inhalations as needed for symptom relief.7 Specific dosing can be found in Table 1. Nearly all current SMART data use formoterol as the LABA because it has a comparable onset of action to albuterol with a longer duration of action.7,11⇓⇓⇓⇓–16 The addition of ICS to relief medication within SMART is advantageous for acute therapy since asthma is an inflammatory disease by nature. While β-agonists address acute bronchoconstriction, they do not mitigate the underlying inflammatory process, putting patients at risk for disease progression. SMART is associated with reductions in severe exacerbations and potential cumulative adverse effects of oral corticosteroids, while adding simplicity of a single inhaler.7,9
Clinical Comparisons Between SMART and Historical ICS-SABA Therapy
Early studies for SMART were conducted in poorly controlled ICS-treated patients comparing budesonide-formoterol SMART with conventional ICS monotherapy plus short-acting β-agonists (SABA) as needed or budesonide-formoterol plus SABA as needed.17 Many studies have shown conflicting results but had design limitations, such as lack of blinding, that may have introduced bias. Later studies more consistently showed signs of improved 12-month outcomes with SMART, including improvements in morning peak expiratory flow, number of reliever-free days, time to first severe exacerbation, and asthma symptom score with severe exacerbations without significant differences in safety outcomes.17 In 2018, a meta-analysis evaluating patients with asthma demonstrated the benefits of SMART over conventional treatment with respect to exacerbation risk, sparking its formal inclusion into the Global Initiative for Asthma (GINA) Report and National Asthma Education and Prevention Program (NAEPP) guidance (Table 2).6,10,18 Notably, the classification of asthma severity differs between these reports. The GINA Report has shifted from using symptoms (SABA frequency, night waking, exacerbations, etc.) exhibited before controller treatment for classification (used by the NAEPP6) to how difficult the disease is to treat5:
Mild: Mild: controlled with as-needed ICS-formoterol or low dose ICS plus as-needed SABA
Moderate: controlled with Step 3 or 4 treatment (low or medium dose ICS/LABA)
Severe: uncontrolled despite high dose ICS/LABA, or requires high dose ICS/LABA to maintain adequate control
Population-Based Single Maintenance and Reliever Therapy (SMART) Recommendations
In clinical practice, the term “mild asthma” should generally be avoided as it can render a false sense of complacency despite continued risk of severe exacerbations.5
Current guidelines advise against extrapolating the benefits of SMART using budesonide-formoterol to other ICS/LABA combinations, such as mometasone-formoterol, as similar efficacy has not been established.5,7,19 Notably, while ICS-formoterol for SMART is guideline-directed, it lacks formal approval by the US Food and Drug Administration (FDA) for this indication, thereby furthering confusion on its implementation.8 The purpose of this article is to summarize the available evidence supporting SMART using ICS-formoterol formulations and facilitate the uptake of this approach to improve asthma care nationwide.
SABA-Only Therapy Is Broken, Let Us Fix It
Historically, SABA alone, most commonly albuterol, were the only quick relief therapies recommended for “mild asthma” (GINA Step 1 or 2).20 However, SABA overuse, defined as using SABA more than twice weekly,1 increases risk of asthma-related emergency department (ED) visits and asthma-related hospitalizations by 25% and 45%, respectively.2 Further, SABA overuse is associated with increased risk of asthma exacerbation and overall mortality.3 Primary care clinicians should use annual and post-ED follow-up visits to discuss the risks of SABA-only regimens.
The known risks of SABA overuse, coupled with the protective effects of regular ICS use,1 prompted updates to the GINA Report in 2019 urging to avoid SABA-only regimens for asthma treatment, which remains the current GINA recommendation.5,18 Instead, primary care clinicians should use symptom-driven or daily inhaled ICS-containing treatment. Unfortunately, ICS adherence has consistently been as low as <50% historically.1,2,4 Many patients continue to opt for SABA-only treatment and thus do not receive the protective anti-inflammatory benefits associated with consistent ICS use. Primary care clinicians should reinforce the benefits of daily ICS to all patients with asthma and advise those taking long-standing SABA-only regimens who are hesitant to switch of the risks of SABA-only treatment. Finally, avoid initiating SABA-only therapy altogether. While not falling under the definition of SMART, GINA notably also prefers the use of ICS-formoterol as a reliever medication in patients with asthma in steps 1 or 2 (those who do not take a maintenance medication). NAEPP guidance does not do the same.5,6 These recommendations derive from a number of ICS-formoterol trials, investigating the use of ICS-formoterol as a reliever medication.11⇓⇓⇓⇓–16
Choose SMART for Adults
Current guidance from the NAEPP and GINA (Table 2)5,6 recommends using ICS-formoterol for SMART over ICS monotherapy plus as needed SABA or ICS-LABA plus as needed SABA for patients with asthma in Steps 3 or 4, who are older than 4 and 5 years, respectively. Table 316,21,22 outlines the evidence behind these recommendations. A 24-week open label, randomized controlled trial (RCT) conducted by Patel and colleagues compared SMART (budesonide-formoterol [200/6 µg per actuation] 2 actuations twice daily plus 1 actuation as needed for symptom relief) with standard treatment (budesonide-formoterol [200/6 µg per actuation] 2 actuations twice daily plus 1 to 2 actuations of salbutamol [100 µg per actuation] as needed for symptom relief) in 303 patients aged 16 to 65 years old with asthma requiring daily ICS for maintenance and with an asthma exacerbation requiring health care presentation and oral corticosteroids or nebulized bronchodilator in the preceding year.21 No statistical differences were observed between groups for the rate of 1 high-use episode (>12 total daily actuations in the SMART group and >16 daily actuations of salbutamol in the standard group). Patients in the SMART group exhibited significantly fewer mean days of high use and fewer number of days with zero actuations of maintenance budesonide-formoterol. In addition, fewer patients in the SMART group had at least 1 severe exacerbation (NNT = 8) and the number of courses of oral corticosteroids per year of follow up was significantly fewer in the SMART group. This study highlights that SMART not only is as safe as historic asthma treatment approaches but also exhibits greater benefit. SMART provides a treatment approach that addresses the risks of SABA-only therapy and the need to increase ICS exposure while simplifying regimens, improving asthma control, and reducing exacerbations.21
Notable Trials Investigating Single Maintenance and Reliever Therapy (SMART) for Asthma
Choose SMART for Children
The benefits of SMART in adults are clear, but asthma often affects children in whom potential dangers of corticosteroids exist. Bisgaard et al.16 conducted a 12-month, double-blind RCT comparing SMART (budesonide-formoterol 80/4.5 µg daily plus additional doses for symptom relief) with fixed combination (budesonide/formoterol 80/4.5 µg daily plus terbutaline 0.4 mg for symptom relief) and fixed-dose budesonide (budesonide 320 µg daily plus terbutaline 0.4 mg for symptom relief) in 341 children ages 4 to 11 years with asthma history ≥6 months and at least 1 clinically important asthma exacerbation in the past 12 months (Table 3). Participants in the SMART group experienced longer time to first exacerbation (P < .001 compared with fixed combination, and P = .02 compared with fixed-dose budesonide) and significantly fewer exacerbations overall compared with the fixed combination (NNT = 5) and fixed-dose budesonide (NNT = 9) groups. Participants in the SMART group also experienced fewer exacerbations requiring medical intervention.16 This research supports the safety and efficacy of SMART in children as young as 4 years old with a history of at least 1 asthma exacerbation.
Exercise-Induced Bronchoconstriction
While SMART may be beneficial in asthma treatment, there is limited data for its use in exercise-induced bronchoconstriction (EIB). A single study in patients >12 years old with EIB showed as needed budesonide-formoterol reduced EIB more than as needed terbutaline alone.22 In addition, combined budesonide-formoterol was noninferior to maintenance budesonide plus as needed terbutaline while exposing participants to significantly lower doses of ICS.22 Further research is needed to fully assess the appropriateness of SMART for EIB. As such, primary care clinicians may offer SABA-only or low dose ICS-formoterol reliever therapy for EIB as a part of patient-centered treatment discussions.5
When Asthma and Chronic Obstructive Pulmonary Disease (COPD) Overlap
Asthma-COPD overlap (ACO) characterizes patients who have persistent airflow restriction in combination with other clinical features of asthma and COPD like significant reversibility and reduced capacity for lung carbon monoxide diffusion, respectively.5 Differentiating between asthma and COPD in patients with long-standing asthma exhibiting persistent airflow limitation can be challenging, especially if other risk factors for COPD, such as smoking, are present. Data suggest that ACO is a disease with a distinct genetic architecture that may show higher response to ICS/LABA than other COPD phenotypes.23 However, the Global Initiative for Chronic Obstructive Lung Disease “GOLD” 2023 Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease does not recognize ACO, but rather classifies asthma and COPD as distinct disorders with common symptoms that may coexist in an individual patient. These guidelines suggest primarily following asthma guidelines in these patients. Newly diagnosed patients with COPD and concurrent asthma using ICS/LABA had a decreased risk of COPD hospitalizations and death versus LABA alone.24 Separately, patients aged ≥66 years with COPD or asthma had decreased morbidity and hospitalizations if treated with ICS alone or in combination.25 As such, consider SMART built on ICS plus LABA and/or LAMA for initial ACO treatment.5,23
Additional Considerations
Adherence
Poor adherence to pharmacotherapy and limited access to care can increase rates of asthma exacerbations and urgent care or ED visits.26 Despite the aforementioned benefits, patient adherence to ICS is underwhelming.4 By incorporating ICS into maintenance and quick relief therapy, SMART improves ICS utilization. Sovani et al. conducted a 6-month, open label RCT comparing maintenance budesonide plus as needed SABA with SMART (budesonide-formoterol 200/6 μg 1 puff once daily and as required) in patients aged 18 to 70 years with clinically diagnosed asthma, currently prescribed 400 to 1000 μg/day of beclomethasone dipropionate or equivalent with poor adherence (<70% of expected ICS prescriptions over the year prior). Participants in the SMART group showed increased cumulative budesonide doses than those assigned to as needed SABA (448 vs 252 μg/day; 95% CI, 113 to 279) and were less likely to withdraw from the study (3 vs 13; P < .01).15 Therefore, primary care clinicians can use SMART to improve ICS adherence in those struggling with daily ICS use.
Cost
Asthma costs the US $81.9 million annually (2008-13) in lost time from missed school or work, ED or urgent care visits, and hospitalizations.27 SABA overuse further increases costs to the health care system.2,28 Administrative claims data show that each additional SABA fill is associated with a 10.8% increase in all-cause costs and a 34.6% increase in asthma-related costs.28 Recent data from Colombia demonstrated that SMART was nearly twice as cost-effective as budesonide-formoterol plus as needed SABA in direct medical costs in patients 5 to 11 years old (2139.60 USD vs 4198.60 USD per year, respectively).29
Further, as aforementioned, ICS-formoterol is currently not FDA-approved as reliever therapy or SMART despite being guideline-directed.8 Therefore, it is also not routinely covered by insurance. Patients who use ICS-formoterol both daily and as needed may require more than 1 inhaler per month and require earlier fills than allowed by payers.
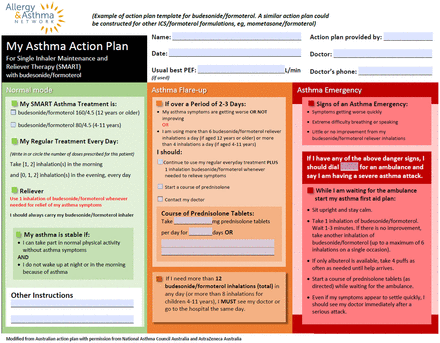
Asthma Action Plans
A main component of asthma management, asthma action plans (AAPs) are formalized documents that help patients self-manage their asthma and improve outcomes.30 AAPs detail maintenance therapy and explicitly outline steps for patients to self-manage flare-ups and recognize exacerbations requiring emergency care.30 Primary care clinicians starting or transitioning patients with asthma to SMART should update AAPs accordingly (Figure 1).
Allergy & Asthma Network SMART Asthma Action Plan.33
Current Challenges and Future Directions
Because SMART studies have been conducted almost exclusively via combined budesonide/formoterol, the utility of SMART using other ICS/β-agonist inhalers is challenging and not recommended. Following the inclusion of SMART as well as the use of ICS-formoterol for symptom relief (steps 1 or 2) into asthma guidelines, the MANDALA and DENALI studies investigated the safety and effectiveness of budesonide-albuterol for asthma symptom relief. These phase-3 RCTs compared fixed-dose combinations of albuterol-budesonide (180-160 μg) [HD-AB], and (180-80 μg) [LD-AB] with budesonide alone (160 μg) [BO], and albuterol alone (180 μg) [AO].31,32 Participants in the HD-AB group showed significantly lower risk of severe asthma exacerbation than those in the AO group (HR 0.74; 95% CI, 0.62-0.89). However, the hazard ratio between the LD-AB and AO groups was statistically insignificant, 0.84 (95% CI, 0.71-1.00).31 Patients receiving fixed dose budesonide-albuterol experienced greater increases in forced expiratory volume in 1 second (FEV1) at 12-weeks. As this product pursues market entry as reliever therapy, we must continue to evaluate the appropriateness of all ICS/β-agonist formulations for use in SMART.
In addition, more data are necessary to assess the long-term risks of SMART. Early studies showed that short-term SMART use was associated with significant increases in sputum and biopsy eosinophilia at 1-year, possibly indicating an inflammatory risk over prolonged use.17 It is important to continue to evaluate and compare guideline-directed therapies as new medications enter the market.
Conclusion
Although a significant shift from traditional asthma management, SMART is a safe and effective asthma treatment approach. Primary care clinicians should not delay starting or transitioning patients aged 4 years or older with asthma requiring a daily maintenance treatment. Despite its effects on immediate symptom relief, SABA-only treatment does not prevent disease progression and should be avoided. Patients receiving ICS-formoterol show improved asthma control and ICS adherence and reduced risk of severe exacerbation over a 12-month period. Long-term data are currently lacking for the risks and benefits of SMART. With new data emerging, it is possible that guidance regarding proper SMART will evolve in the coming years.
Notes
This article was externally peer reviewed.
Funding: No sources of funding were utilized for this article.
Conflict of interest: The authors report no potential conflict of interest relevant to this article.
To see this article online, please go to: http://jabfm.org/content/37/4/745.full.
- Received for publication December 6, 2023.
- Accepted for publication March 11, 2024.