Abstract
Objective: The objective of this study was to compare the performance of the US Preventive Services Task Force (USPSTF) recommended WHO Fracture Risk Assessment Tool (FRAX) threshold score of 9.3% (calculated without femoral neck bone density) with the Simple Calculated Osteoporosis Risk Estimate (SCORE), Osteoporosis Self-Assessment Tool (OST), and the Osteoporosis Risk Assessment Instrument (ORAI) to identify osteoporosis in younger women.
Methods: We conducted a retrospective review of women ages 50 to 64 years who underwent dual-energy radiographic absorptiometry (DXA) at our institution over a 6-month period. Scores for the FRAX, ORAI, OST, and SCORE tools were calculated using various thresholds: FRAX ≥9.3%, SCORE ≥6, OST <2, and ORAI ≥9. Sensitivity, specificity, and area under the receiver-operating characteristic curve for detection of densitometric osteoporosis by DXA for each tool were compared.
Results: A total of 290 women were identified. Of these, 284 (97.9%) were white, and the mean ± standard deviation age was 56.6 ± 3.4 years. Fifty (17.2%) had osteoporosis of the lumbar spine and/or femoral neck on DXA. Sensitivity, specificity, and area under the receiver-operating characteristic curve for identifying densitometric osteoporosis at the femoral neck and/or spine were 36%, 73%, and 0.55 for FRAX; 74%, 42%, and 0.58 for SCORE; 56%, 69%, and 0.63 for the OST; and 52%, 67%, and 0.60 for the ORAI, respectively.
Conclusions: DXA screening based on the USPSTF–recommended FRAX threshold score of 9.3% has a low sensitivity to identify densitometric osteoporosis in women ages 50 to 64. Lowering the threshold score would increase sensitivity but would also increase the number of women sent for screening DXA. Use of the validated SCORE tool would improve sensitivity to identify osteoporosis in this age group.
The National Osteoporosis Foundation (NOF), US Preventive Services Task Force (USPSTF), American Association of Clinical Endocrinologists, American College of Obstetricians and Gynecologists, and American College of Preventive Medicine all recommend that women 65 years and older should be screened for osteoporosis.1⇓⇓⇓–5 For women ages 50 to 64 years, most guidelines recommend assessing for clinical risk factors for osteoporosis and considering bone mineral density (BMD) testing in women at increased risk.4⇓–6 A 2011 update to the USPSTF screening guidelines recommended that women younger than 65 be screened for osteoporosis with dual-energy radiographic absorptiometry (DXA) if their 10-year risk of a major osteoporotic fracture (MOF) is equal to or greater than that of a 65-year-old white woman without additional risk factors; this was listed as a grade B recommendation.3 To determine fracture risk, the USPSTF suggests the use of the World Health Organization Fracture Risk Assessment Tool (FRAX)7 based on the rationale that it has been extensively validated, is based on easy-to-determine clinical factors, and is readily available for widespread use.3 Using FRAX, the 10-year MOF risk of a 65-year-old white woman without additional risk factors is estimated to be 9.3%.3 Thus the USPSTF currently recommends that all women ages 50 to 64 years with a calculated FRAX 10-year MOF risk of ≥9.3% be screened for osteoporosis.
FRAX was developed as a fracture risk assessment tool to guide treatment decisions rather than as an osteoporosis risk assessment tool; however, current USPSTF guidelines regarding women in the 50- to 64-year-old age group promote use of FRAX to assess the need for BMD screening (ie, the guidelines promote the use of FRAX as an osteoporosis screening tool rather than as a fracture risk assessment tool, as originally intended). Previous investigations suggest that the USPSTF FRAX threshold score of an MOF of ≥9.3% to perform DXA in this age group has a low sensitivity for detecting densitometric osteoporosis.8,9 Clinical prediction rules that were created and validated to assess the likelihood of osteoporosis include the Osteoporosis Risk Assessment Instrument (ORAI), Osteoporosis Self-Assessment Tool (OST), and the Simple Calculated Osteoporosis Risk Estimation (SCORE).10⇓–12 The purpose of our study is to evaluate the ability of these 4 clinical prediction rules to identify women with osteoporosis among a cohort of women ages 50 to 64 years, and to compare the sensitivity and specificity of the current USPSTF FRAX score threshold of an MOF of 9.3% with the validated ORAI, OST, and SCORE clinical prediction rules for osteoporosis detection.
Methods
We retrospectively identified all women ages 50 to 64 years empaneled with a primary care provider at the Mayo Clinic in Rochester, Minnesota, who underwent DXA of the hip and/or lumbar spine over a 6-month period. Demographic and relevant clinical data were collected from the medical records to determine risk profiles with the FRAX, ORAI, OST, and SCORE tools. Subjects were excluded if they were previously treated with any of the following medications that could alter BMD: bisphosphonates, calcitonin, selective estrogen receptor modulators, or parathyroid hormone analogs. Women with a history of or current estrogen use, or a history of fragility fractures, were not excluded because these are risk factors included in 2 of the tools. The specific clinical risk factors for each clinical prediction tool are shown in Table 1.
Scores for the FRAX, ORAI, OST, and SCORE tools were calculated and the sensitivity, specificity, area under the receiver-operating characteristic curve, positive predictive value, and negative predictive value to detect densitometric osteoporosis (by DXA) were calculated using JMP Pro 9.0.1 (SAS Institute, Cary, NC) and were compared for each screening tool. Because the USPSTF guidelines recommend using FRAX to identify women to be screened for osteoporosis, the FRAX calculation was performed without using any bone density measurements. Osteoporosis was defined as a BMD T-score of ≤−2.5 at either the femoral neck and/or the lumbar spine (average of ≥2 vertebrae).
This study was approved by the Mayo Clinic institutional review board.
Results
A total of 290 women were identified as eligible for evaluation. Of these, 284 (97.9%) were white, and the mean ± standard deviation age was 56.6 ± 3.4 years. A total of 180 (62%) had a body mass index ≥25.0. The majority of women had no history of a fragility fracture. Nineteen (6.6%) were identified as having osteoporosis of the femoral neck, 41 (15.1%) had osteoporosis of the lumbar spine, and 50 (17.2%) were identified as having osteoporosis at either the femoral neck, the lumbar spine, or both. Characteristics of the study population are shown in Table 2.
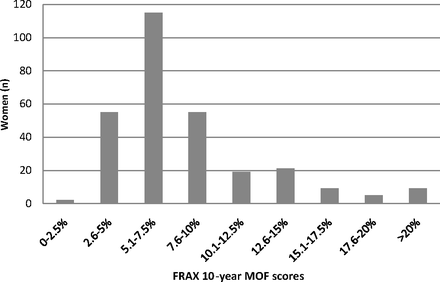
The distribution of FRAX scores in the group is shown in Figure 1. The majority of women had a FRAX score between 2.6% to 10%, and 81 women (27.9%) had a FRAX score ≥9.3%. Of the 209 women who had a FRAX score <9.3%, 32 (15.3%) had densitometric osteoporosis. Of the 50 women with densitometric osteoporosis on DXA, 18 (36%) had a FRAX score ≥9.3%.
Distribution of the estimated 10-year major osteoporotic fracture risk (MOF) among 290 women, based on the World Health Organization Fracture Risk Assessment Tool (FRAX; calculated without femoral neck bone density).
Sensitivity, specificity, and area under the receiver-operating characteristic curve for identifying densitometric osteoporosis at the femoral neck and/or spine for the various prediction tools at recommended thresholds are shown in Table 3. To obtain a sensitivity of approximately 90% (which was the approximate sensitivity used to choose threshold cutoff values in the development of the OST, ORAI, and SCORE osteoporosis prediction tools)10⇓–12 requires decreasing the FRAX threshold to 4.7%. To obtain sensitivities of approximately 90% requires lowering the threshold for ORAI and SCORE and increasing the threshold value for OST (Table 4). Our data further suggest that a FRAX threshold of 6.8% would optimize the trade-off between sensitivity (66%) and specificity (52%). The numbers of women who would be sent for screening using each tool based on the recommended thresholds and thresholds that give sensitivities of approximately 90% are shown in Tables 3 and 4, respectively. For FRAX, 243 (84%), 149 (51%), and 81 women (28%) would be referred for DXA at thresholds of 4.7%, 6.8%, and 9.3%, respectively.
Discussion
The OST, SCORE, and ORAI tools were all developed to identify the risk of osteoporosis in women to help guide the decision of whether to screen for osteoporosis, whereas FRAX was developed to determine the risk of osteoporotic fractures and guide treatment decisions.10⇓–12,14 Despite this important distinction, the 2011 USPSTF recommendations promote FRAX as an osteoporosis risk assessment tool to decide which women under age 65 years should be screened for osteoporosis using DXA.3 In our study, however, this strategy was not very sensitive, detecting only about one third of women with densitometric osteoporosis.
In our study population the SCORE tool, at the recommended threshold of ≥6, had significantly greater sensitivity for detecting osteoporosis in women 50 to 64 years old than the current USPSTF recommended FRAX threshold of 9.3%. Similarly, in a study of women ages 50 to 64 years, Crandall et al8 found that both OST and SCORE (ORAI was not evaluated) had higher sensitivity to detect osteoporosis at the femoral neck than the current FRAX threshold of 9.3%. However, they did not evaluate the sensitivities and specificities of the various tools to identify osteoporosis at the lumbar spine. The investigators noted that the currently recommended USPSTF FRAX threshold of 9.3% for screening only identified about one third of women in this age group with osteoporosis at the femoral neck,8 which is similar to our finding for identifying osteoporosis at the femoral neck and/or lumbar spine.
The recommendation to screen women between 50 and 64 years of age if their FRAX 10-year predicted risk of an MOF is ≥9.3% may seem intuitive given that this risk is equivalent to that of a 65-year-old white woman with no other risk factors for fracture and for whom essentially all guidelines would recommend screening. However, if the goal of osteoporosis risk assessment is to identify densitometric osteoporosis, it seems that more liberal threshold values may be needed to achieve similar sensitivities for osteoporosis detection in younger women compared with older women. In a study comparing a population similar to ours (all white women aged 45 to 64 years old with a mean age of 56 years) with women ≥65 years old, the authors also found that to achieve the same sensitivity (approximately 90%) for osteoporosis detection, the threshold scores to screen with the OST, ORAI, and SCORE all needed to be liberalized for the younger age cohort compared with the cohort of women aged ≥65 years.15 This finding is supported by our study. Though the populations used to develop ORAI, OST, and SCORE included women younger than age 65 years, the average age cited (ranging from 61.3 to 63.5 years) was older than the average age (56.6 years) of our study population, which might provide additional support for the finding that younger women require different assessment threshold scores than women ≥65 years to achieve similar sensitivities for osteoporosis clinical prediction tools.11,12,16
Achieving a sensitivity of 90% (the approximate sensitivity used to choose threshold values for OST, ORAI, and SCORE when they were developed)10⇓–12 requires decreasing the FRAX MOF threshold for screening to 4.7%, which would decrease the specificity to 17.5%. Using a FRAX threshold of 4.7% would result in 84% of women in this age group being sent for DXA, whereas the use of SCORE with a threshold ≥6 (the threshold recommended for screening) would give a reasonably high sensitivity of 74% and result in 60% of women being recommended for screening.
One benefit of the use of FRAX to guide osteoporosis assessment, as listed in the USPSTF recommendation statement, is that it “relies on easily obtainable clinical information, such as age, body mass index (BMI), parental fracture history, and tobacco and alcohol use; its development was supported by a broad international collaboration and extensively validated in 2 large US cohorts; and it is freely accessible to clinicians and the public.”3 However, SCORE is available as an online calculator,17 and it also involves easily obtainable clinical information. In fact, SCORE requires the entry of less clinical information (6 clinical risk factors compared with 11 in FRAX, excluding femoral neck density). In addition, SCORE was developed specifically to assess osteoporosis risk prediction, whereas FRAX was developed for fracture risk prediction.
Our study has several limitations, including the retrospective design and the fact that the study population consisted of women already selected for osteoporosis screening by their provider. However, even in this preselected population (in whom there presumably was some risk factor that prompted the provider to order DXA), the sensitivity of the USPTF-recommended FRAX threshold of 9.3% was low. Another potential limitation is that our study population was primarily white, but this racial group has the highest risk for osteoporosis and thus would be expected to have a higher prevalence of osteoporosis compared with other groups. Last, our study defined the presence of osteoporosis based solely on densitometric criteria. Therefore the detection rate at any given FRAX threshold does not necessarily reflect the detection rate for all cases of osteoporosis and situations where NOF guidelines would recommend treatment (eg, women with a history of a hip or vertebral fracture, or osteopenia with a FRAX predicted 10-year probability of an MOF ≥20%, and/or predicted 10-year hip fracture probability ≥3%) in our population.
The goal of osteoporosis screening is to identify women with a T-score ≤−2.5 so that therapy can be considered with the ultimate goal of decreasing the incidence of fractures. While basing the threshold for treatment for women without a history of fragility fracture at the fracture probability (by FRAX) that is equal to that of age-matched women who have had a fragility fracture has been proposed as an alternative approach to preventing fractures,18⇓–20 current NOF guidelines recommend treatment based on DXA bone density results (osteoporosis or osteopenia plus estimated 10-year hip and MOF risks, as described above) or a history of hip or vertebral fracture. Thus, with current NOF treatment guidelines, the primary value of osteoporosis assessment tools is to identify women who are likely to have densitometric osteoporosis and qualify for treatment. In younger postmenopausal women, bisphosphonates have been shown to increase bone density and decrease biochemical bone turnover markers.21,22 These surrogate markers provide indirect support for bisphosphonate's ability to decrease fracture risk in this age group.23,24 While we are not aware of a trial that has evaluated the fracture prevention efficacy of bisphosphonates specifically in younger postmenopausal women, the Fracture Intervention Trial included women ages 55 to 65 years, and a subgroup analysis revealed that alendronate is effective for fracture risk reduction in this younger age group.25,26
Conclusion
Applying the USPTF-recommended FRAX MOF threshold of 9.3% to assist in selecting younger women for bone density testing would fail to identify a large proportion of women ages 50 to 64 years who have osteoporosis, and the opportunity for fracture prevention would be missed. Lowering the FRAX MOF threshold score that would prompt DXA screening to 4.7% would increase the sensitivity to detect and diagnose osteoporosis in women in this age group, though it would also increase the number of women recommended for screening. Use of SCORE at the recommended threshold of ≥6 is an alternative strategy that requires less clinical information and could be used to improve the sensitivity of detecting osteoporosis in this younger age group.
Acknowledgments
The authors thank Stephanie Quigg and Julie Maxson for assistance with data collection.
Notes
This article was externally peer reviewed.
Funding: This study was supported by departmental funds.
Conflict of interest: none declared.
- Received for publication July 28, 2015.
- Revision received October 21, 2015.
- Accepted for publication October 29, 2015.