Abstract
Family physicians play a crucial role in the management and ongoing care of patients with Alzheimer disease (AD). This article reviews the effects of nonpharmacologic and pharmacologic interventions on the functional abilities and behavior of patients with dementia and how these can be implemented into clinical practice. Nonpharmacologic interventions are recommended as the initial strategy for managing problematic behaviors. Strategies for improving behavior include ensuring that the patient's environment is safe, calm, and predictable; removing environmental stressors; and identifying and avoiding situations that agitate or frighten the patient. Simple interventions include redirecting and refocusing the patient, increasing social interaction, establishing regular sleep habits, eliminating sources of conflict and frustration, and establishing rewards for successes. The effectiveness of long-term behavioral management is largely dependent on the caregiver; as such, it is important to assess the role and needs of the caregiver.
Because currently available therapies cannot reverse the pathologic processes of AD, the primary objective of pharmacotherapy is to preserve cognitive and functional ability, minimize behavioral disturbances, and slow disease progression. Cholinesterase inhibitors represent first-line therapy for patients with mild to moderate AD, whereas a glutamate N-methyl D-aspartate antagonist is used in the treatment of moderate to severe AD. Looking forward, there are a number of therapies in development aimed at modifying the disease course; these include amyloid-lowering drugs, τ-based and neuroprotective approaches, acetylcholine agonists, and mitochondrial inhibitors.
The Role of the Family Physician in Treating Dementia
Primary care is the point of first medical contact for people with dementia and hence the cornerstone of ensuring early detection, timely intervention, and effective ongoing management.1 Inadequate detection and poor management have been reported globally,2,3 leading to people with dementia and their families being denied optimal pharmacologic4 and psychosocial intervention.5 Alzheimer disease (AD), the most common cause of dementia worldwide, is a complex disorder that warrants a multidimensional approach with regular monitoring of the patient for increasing cognitive, functional, and behavioral challenges. Management consists of both pharmacologic and nonpharmacologic interventions as well as referrals to social service agencies and support resources, such as the Alzheimer's Association (www.alz.org). The family physician plays a key role in linking the family to community resources and other health care and social service providers who will help implement the overall care plan.6,7 Physicians also play a key role in coordinating the invaluable support network of nurse practitioners, physician assistants, social workers, and medical assistants. Moreover, the family physician can assist in maintaining the physical health of patients with dementia, for example, assisting with the evaluation and treatment of visual and hearing defects, which are more common with aging. Such assistance can help directly and indirectly in the management of dementia.
For the purpose of this review, an electronic search of English-language articles (without time limits) was performed using PubMed and MEDLINE. The primary research parameters were Alzheimer's disease, diagnosis, therapy, treatment, and therapeutic. Original research articles, reviews, and other articles of interest were reviewed, and the most important information was identified. This review provides a summary of these findings as well as practical advice for the busy clinician.
Managing Cognitive, Memory, and Functioning Problems
Goals of Therapy and Likely Outcomes
The management of a patient with AD is a complex and evolving task because the natural history of AD is one of progressive decline; patients' cognitive, physical, and social functions gradually deteriorate.8 One of the key aspects of optimal management of dementia is realistic expectations for therapeutic outcomes, including treatment effects and potential outcomes; it is, therefore, imperative that the family physician is aware of these issues and discusses them with both the patient and caregiver.9 To be effective, interventions for patients with dementia ideally will improve functional status to a level that is detectable by caregivers or health care providers.
In clinical trials, the Alzheimer's Disease Assessment Scale, Cognitive Subscale (ADAS-Cog), a 20-minute, 11-item, 70-point scale that tests memory, language, orientation, and praxis, is often used to determine rate of cognitive decline. The total score ranges from 0 to 70, with a high score indicating greater impairment.10,11 Because of the progressive nature of AD, there may be brief plateaus during the illness; however, the decline is fairly consistent, tending to increase or accelerate as patients enter the moderate stage.12 Therefore, any “improvement” from an intervention for dementia must take this into account. As such, “improvement” can be defined as a reduction in rate of decline.8 For example, patients with mild dementia experience an average rate of decline of ≤5 ADAS-Cog points,8 and slowing this decline by 2 to 3 ADAS-Cog points over a year could mean a delay of up to 7 months in disease progression. In contrast, patients with moderate dementia (ADAS-Cog score >15 but <55) experience an average decline in cognition of 7 to 11 ADAS-Cog points (2–4 Mini Mental State Examination [MMSE] points) annually.13 Therefore, for people with moderate dementia, slowing decline by 2 to 3 ADAS-Cog points per year could mean a delay of 2 to 5 months in disease progression. In general, cholinesterase inhibitors (ChEIs) do not delay ultimate progression of AD by more than 6 to 7 months.
Nonpharmacologic Interventions
An increasing number of nonpharmacologic therapies are now available for people with dementia, including behavioral therapy, reality orientation, art therapy, music therapy, complementary therapy, aromatherapy and bright-light therapy, as well as cognitive behavioral therapies. There are several areas of overlap between these therapies and each approach is rarely used in isolation14; it is therefore useful for clinicians to be familiar with several of these approaches to enable a combination of treatments to be tailored to individual requirements.15 Therapy is now directed toward person-centered forms of care and greater attempts are made to understand the individual's experience of dementia and to employ strategies to improve the person's quality of life (QoL).15 Individualized nonpharmacologic interventions include self-affirming exercises, such as reminiscence therapy, and structured socialization, such as pet therapy and viewing family videotapes.16 The efficacy of these interventions has been demonstrated in both small and larger studies.17
Dietary Supplements
Several nutrient deficiencies are known to be risk factors for AD. Evidence suggests that consumption of fish with high fat content and marine omega-3 polyunsaturated fatty acid decreases the risk of cognitive impairment and dementia.18 It is, therefore, not unusual in clinical practice to encounter patients and caregivers inquiring about dietary recommendations for lowering the risk of dementia. However, to date, there are no clinical trials to support a recommendation of dietary and supplemental omega-3 polyunsaturated fatty acid for the sole purpose of preventing cognitive impairment or dementia.18 Nevertheless, it is not unwarranted to encourage adequate consumption of fatty fish as part of general dietary recommendations that may also confer benefits of reducing the risk of stroke and heart disease.19
There has been recent attention regarding the health benefits of curcumin (found in the commonly used Asian spice, turmeric) in AD. In animal studies, low-dose curcumin effectively disaggregates β-amyloid and prevents fibril and oligomer formation, supporting the rationale for curcumin use in clinical trials preventing or treating AD.20 Indeed, a phase II clinical trial with patients with moderate to severe AD is ongoing, designed to determine whether curcumin can slow cognitive deterioration.21
Finally, recent studies from both the United States and Europe have suggested that vitamin D deficiency may be associated with increased odds of cognitive impairment in older persons.22,23 Indeed, results from a study in the United States in which cognitive impairment was assessed using measures of immediate and delayed verbal memory, orientation, and attention reported a link between vitamin D deficiency and cognitive impairment in 3325 adults aged >65 years.22 The multivariate adjusted odds ratios (95% confidence interval) of cognitive impairment in patients who were vitamin D insufficient (≥50 < 75 nmol/L), deficient (≥25 < 50 nmol/L), and severely deficient (<25 nmol/L) compared with those sufficient (≥75 nmol/L) were 0.9 (0.6–1.3), 1.4 (1.0–2.1), and 3.9 (1.5–10.4), respectively (P for linear trend = .02), suggesting that vitamin D deficiency is associated with increased odds of cognitive impairment among the elderly population. Similar findings also have been reported in a European study.23 Although further exploration of a possible causal relationship between vitamin D deficiency and cognitive impairment is warranted, these findings raise important new possibilities for treatment and prevention of cognitive decline in patients with AD.
Medical Foods
Medical foods as a class of intervention alternatives are not well known to most clinicians but are a growing area. Medical foods are a special category of US Food and Drug Administration (FDA)–regulated agents intended to provide specific nutritional requirements for patients with certain diseases; they can, therefore, provide an additional supplement in a comprehensive therapeutic regimen for patients with AD. Products being marketed currently or developed in the United States for the management of dementia include Caprylic triglyceride (Axona, Accera, Inc., Broomfield, CO) and Souvenaid (Nutricia Advanced Medical Nutrition, Schiphol, The Netherlands).
Axona has been developed for the clinical dietary management of the metabolic processes associated with mild to moderate AD. It is a formulation of caprylic triglyceride, a medium-chain triglyceride that is metabolized to ketone bodies, predominantly β-hydroxybutyrate, a common metabolic substrate that is produced normally by the body for neurons in starvation states where glucose is less available.24 A double-blind crossover study conducted in patients with AD or mild cognitive impairment demonstrated that Axona therapy was associated with significant improvements in ADAS-Cog; however, the effect was seen only in patients who were not carriers of apolipoprotein E ε4.24 Similar results were reported in a 90-day, randomized, placebo-controlled study in patients with mild to moderate AD.25 Significant gastrointestinal side effects have been associated with Axona, and slow titration of the product is being recommended.
Souvenaid (food) combines omega-3 fatty acids, choline, uridine monophosphate, and a mixture of antioxidants and B vitamins.26 In a randomized, controlled trial involving more than 200 patients with mild AD, Souvenaid was well tolerated and improved memory compared with placebo.27
Pharmacologic Interventions
There are currently no means of reversing the pathologic processes of AD. Currently available medications do not halt the underlying degenerative process but can slow disease progression and therefore delay symptomatic decline.28 The specific goals of therapy are to preserve cognitive and functional ability, minimize behavioral disturbances, and slow disease progression with maintenance of patients' and caregivers' QoL.29 Nevertheless, realistic expectations of treatment outcomes are needed because the impact for most patients is likely to be modest and temporary, with not every patient responding to treatment. The main benefit of pharmacotherapy is an attenuation of decline over time rather than an improvement in cognitive or behavioral symptoms. It is important to discuss this point with patients and their families, who may expect improvement rather than relative stability.30 Failure to do so often will result in patient and family dissatisfaction with prescribed therapies and the risk of discontinuation. Beneficial response to a ChEI (ie, delayed deterioration of cognitive or behavioral problems) can be determined from the physician's global assessment of the patient, the primary caregiver's report, a neuropsychologic assessment or mental status questionnaire, or evidence of behavioral or functional changes.7
Four drugs are commonly used for treating AD: 3 ChEIs approved for mild to moderate disease, one of which also is approved for severe AD, and a glutamate N-methyl D-aspartate (NMDA) antagonist approved for moderate to severe disease (Table 1).30
Mild to Moderate Disease
Since the introduction of the first ChEI in 1997, most clinicians would consider these agents to be first-line pharmacotherapy for mild to moderate AD.11 Four ChEIs are currently available: tacrine (Cognex, Shionogi, Inc., Atlanta, GA); donepezil (Aricept, Eisai Co, Ltd., Woodcliff Lake, NJ); rivastigmine (Exelon, Novartis Pharmaceuticals Corp., East Hanover, NJ); and galantamine (Reminyl, Ortho-McNeil Neurologics, Titusville, NJ). Tacrine is not commonly used because of a poor tolerability profile and low oral bioavailability, and it is, therefore, excluded from this discussion.31 ChEIs raise acetylcholine levels in the brain by inhibiting acetylcholinesterase.6 Despite minor variations in their mode of action there is no evidence to suggest any difference in efficacy between the 3 commonly used ChEIs.11 Likewise, the tolerability profile is similar between the ChEIs for the oral formulations. However, the 10-cm2 rivastigmine patch has shown efficacy similar to oral rivastigmine formulations, but with approximately two-thirds fewer reports of nausea and vomiting, with adverse event (AE) rates similar to those of placebo32 (Table 1). AD often is accompanied and worsened by malnutrition, and weight loss is a frequent complication of AD, occurring in approximately 40% of patients at all stages.33 Donepezil, rivastigmine, and galantamine cause a broad spectrum of AEs, of which nausea, vomiting, diarrhea, and weight loss are the most common.34,35
There continues to be debate regarding the extent of the benefits achieved with ChEIs. Although some assert that the most that can be achieved with ChEIs is symptom modification,11 others consider these agents to have disease-modifying effects.36 In one study, after discontinuation of therapy, rivastigmine-treated patients showed less deterioration in cognitive function compared with placebo-treated patients, suggesting an effect on disease progression.37 In another study, donepezil treatment slowed progression of hippocampal atrophy compared with untreated patients, suggesting a neuroprotective effect of donepezil in AD.38 However, these early observations require confirmation, and, at present, the ChEIs generally are considered symptomatic medications.
A systematic analysis of double-blind, placebo-controlled trials of ChEIs demonstrated treatment effects ranging from a 1.4- to 3.9-point improvement at 6 months and 1 year, in the midrange of the 70-point ADAS-Cog scale.11 In clinical trials, a change of 4 points is considered clinically significant for patients with mild to moderate dementia.39,40 As such, the symptomatic improvements observed are modest and of debatable clinical significance, despite being statistically significant.35 In a meta-analysis of 16 double-blind, placebo-controlled trials of ChEIs composed of almost 8000 patients, the numbers needed to treat for one additional patient to benefit were 7 for stabilization or better, 12 for minimal improvement or better, and 42 for marked improvement.41 Although the numbers needed to treat seem favorable, uncertainty remains regarding the clinical relevance of these outcomes and the duration of the apparent benefit because the majority of trials reviewed were of less than 26 weeks' duration.
In addition to their effects on cognition, these agents also have demonstrated beneficial effects on measures of behavior, activities of daily living (ADLs), and global patient function. A recent meta-analysis that analyzed clinical results from 29 randomized, placebo-controlled trials of patients with mild to moderate AD found that ChEI therapy was associated with significant modest benefits in terms of neuropsychiatric and functional outcomes.42 Current guidelines acknowledge that preventing or delaying further loss of ADL function is an important goal of AD therapy43 and that the benefits of ChEIs may be diminished when treatment is delayed.28 Significant preservation of ADL function has been observed with donepezil, galantamine, and rivastigmine compared with placebo.29
ChEIs also have been shown to reduce AD caregiver burden: in patients with moderate to severe AD, donepezil treatment for 24 weeks significantly reduced caregiver time spent assisting patients with basic and instrumental ADLs (−52 minutes/day; P < .005).44 A small study has demonstrated that rivastigmine treatment reduces caregiver time spent assisting with ADLs (up to 690 hours over 2 years).45 Longer periods of treatment with ChEIs also decrease the risk for nursing home placement.46,47 A retrospective analysis of a large US medical claims database showed that over a 27-month follow-up period, more patients who were not treated with ChEIs were placed in nursing homes (11.0%) than were those who received either rivastigmine (3.7%) or donepezil (4.4%).47 These studies suggest that ChEIs enable patients to live longer in community settings with associated personal, social, and economic benefits.29
Memantine (Namenda, Forest Pharmaceuticals, St. Louis, MO) is sometimes used to treat patients with less severe disease, despite its use in early AD not being supported by the FDA. Although memantine has been reported to improve cognition, global status, and behavior in patients with mild to moderate AD,48 its mechanism of action would suggest that it does not have a place in early AD. Memantine is not a ChEI; it is a low- to moderate-affinity, noncompetitive (channel blocking), NMDA-receptor antagonist that seems to block pathologic neural toxicity associated with prolonged glutamate release.49 Blockade of NMDA receptors by memantine could confer disease-modifying activity in AD by inhibiting the “weak” NMDA receptor–dependent excitotoxicity that contributes to the neuronal loss underlying the progression of dementia.49 As such, memantine is not effective until weakened neurons become vulnerable to glutamate-induced excitotoxicity, and therefore it cannot substitute for ChEIs because of its inability to enhance cholinergic neurotransmission required for memory and learning.49
Moderate to Severe Disease
Memantine is approved for the treatment of moderate to severe AD on the basis of a study in which patients with moderate to severe AD who received 20 mg memantine monotherapy showed less decline in cognition and function while maintaining good tolerability after 6 months compared with those who received placebo.50 The ChEI donepezil also recently has been approved for use in severe AD.
Recently, donepezil 23 mg/day has been approved for the treatment of moderate to severe AD. Results from a 24-week, randomized, double-blind study reported that donepezil 23 mg/day was associated with greater benefits in cognition (as assessed by the Severe Impairment Battery) compared with donepezil 10 mg/day, although the between-treatment difference in the Clinician's Interview-Based Impression of Change plus Caregiver Input Scale was not significant. The most commonly reported side effects with donepezil 23 mg/day were nausea vomiting, and diarrhea, which occurred at a higher incidence than with donepezil 10 mg/day.51
Combination therapy of a ChEI and memantine is rational from a pharmacologic perspective because the agents have different mechanisms of action. In a randomized controlled trial, patients with moderate to severe AD who were already receiving donepezil derived significant benefit from the addition of memantine in terms of cognition, ADLs, global outcome, and behavior.52 There are also economic benefits associated with the addition of memantine to donepezil treatment for patients with advanced AD. A recent study demonstrated improvement in clinical outcomes plus cost savings associated with the use of memantine.53 In a study by Tariot et al,52 the incidence of nausea was substantially lower in patients receiving memantine add-on therapy compared with those receiving donepezil monotherapy. The safety and tolerability of combining rivastigmine capsule and memantine also has been studied in a 26-week, prospective, open-label study of patients with moderate AD.54 The combination was found to be both tolerable and safe, with a reduced incidence of gastrointestinal-related AEs compared with those documented in the US prescribing information for rivastigmine, suggesting that this beneficial effect of memantine may be applicable across ChEIs.54
Adjuncts to Pharmacotherapy for Improving Cognitive Function
A recent study has demonstrated that ChEI-treated patients with early AD who received psychosocial support plus cognitive-motor intervention (CMI) had additional mood and cognitive benefits over those experienced by ChEI-treated patients who received psychosocial support alone. The CMI consisted of a 1-year structured program of 103 sessions, including reality orientation techniques, cognitive exercises, training of ADLs, and psychomotor exercises. Cognitive exercises were designed to stimulate memory, attention, language, visuospatial abilities, calculation, and frontal/executive functions. The ADL training was related to the particular cognitive function stimulated at each session (eg, money handling was trained after calculation exercises). The results showed that patients in the CMI group maintained cognitive status at 6 months, whereas patients in the control group had significantly declined by that time. In addition, more patients who received CMI maintained or improved their affective status after 1 year (CMI group, 75%; control group, 47%).55
Treatment Guidelines
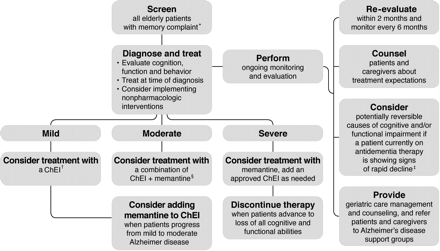
In 2006, a panel of leading experts published recommendations for best practice in the treatment and management of AD. These recommendations were developed in an effort to address issues surrounding early diagnosis, treatment, and care management of AD, as well as societal and managed-care implications.56 An algorithm was created to assist providers with the appropriate utilization of therapy and care management (Figure 1). This algorithm recommends initiating ChEI therapy in patients with mild AD and using combination therapy with a ChEI and memantine for patients who progress from mild to moderate AD. Alternatively, global guidelines recommend that patients who continue on the drug should be reviewed every 6 months by MMSE score and global, functional, and behavioral assessment.9 Treatment should be continued only while the patient's MMSE score remains ≥10 points and their global, functional, and behavioral condition indicates that the drug is having a worthwhile effect. In patients with moderate to severe AD (MMSE score <12), treatment with memantine can be considered, alone or in combination with a ChEI.9
Treatment and management of Alzheimer disease. *Memory complaint may be raised by family or caregiver. All patients aged ≥75 years should be screened regardless of clinical presentation. †Cholinesterase inhibitors (ChEIs) are included for mild to moderate Alzheimer disease, excluding donepezil, which is indicated for mild, moderate, and severe Alzheimer disease. ‡Possible causes include medical comorbidities, the effects of other drugs, behavioral disturbances, or delirium. §Memantine is indicated for the treatment of moderate to severe Alzheimer disease. (This treatment algorithm is derived from recommendations published in Ref. 56. Reproduced with permission from RG Stefanacci. Reinforcing the value of combination therapy to treat moderate to severe alzheimer's disease. Phys Week 2009;26(9). © 2009 Physician's Weekly, LLC.)
Managing Mood Disorders and Behavior Problems
Traditionally, cognitive function has been the main focus of interest in treatment and research of people with dementia. It is becoming increasingly recognized, however, that noncognitive symptoms are those that are most disturbing to families and caregivers and may seriously impact not only the patient's well-being, but also the family's, caregivers', and providers' approaches to managing the patient.15 The most common symptoms are agitation, aggression, mood disorders/behavioral disturbance, apathy, depression, psychosis and hallucinations, with sexual disinhibition, elation/euphoria, appetite and eating disturbances, and abnormal vocalizations occurring less frequently.15,57 These have been grouped together under the umbrella term behavioral and psychological symptoms of dementia by the International Psychogeriatric Association.58 As the disease progresses, these symptoms become predominant problems59 and impose an enormous toll, both emotionally and financially.17 They are also a common reason for institutionalization of people with dementia and they increase the burden and stress of caregivers.55,57,60
Nonpharmacologic Interventions
Nonpharmacologic interventions are recommended as the most appropriate initial strategy for managing inappropriate behaviors in dementia because (1) they address the psychosocial/environmental underlying reason for the behavior, and (2) they avoid the limitations of pharmacologic interventions, namely, adverse side effects, drug–drug interactions, and limited efficacy.15,17,61 Increased involvement of caregivers often has a secondary benefit of providing overburdened caregivers with an opportunity to receive support, information, and skills. Furthermore, environmental factors (eg, confusing or noisy surroundings) or interpersonal factors (eg, arguing with the patient) are often the primary triggers of behavior problems. Attention to these factors through nonpharmacologic approaches can be effective in alleviating or preventing behavioral problems in individuals with dementia and should be considered first.6,59,61 A recent consensus statement recommended that all treatment approaches start with rigorous attempts to identify any reversible causes of these behaviors and alleviate these factors16 by modifying the physical and interpersonal environments.62,63 Common triggers of agitation and aggression include pain, fecal impaction, medical illness, boredom, loneliness, depression, and social and environmental stressors. Unfortunately, in practice, pharmacologic approaches involving neuroleptic or other sedative medication are often used as the first-line treatment, despite the modest evidence of efficacy from clinical trials in which high placebo response rates frequently are seen.15,64
Patients with AD function best in an environment that is safe, calm, and predictable, and their caregivers require ongoing support and education to develop realistic expectations throughout the course of the illness.30 Caregivers can be taught strategies to reduce agitation and anxiety in patients with dementia.65 One such strategy utilizes the 3 Rs approach (repeat, reassure, and redirect), whereby the caregiver repeats an instruction or answer to a question as needed and redirects the patient to another activity to divert attention from a problematic situation. A predictable routine also is important and may avert certain behavioral problems. For example, scheduled toileting or prompted voiding can reduce urinary incontinence.6 Training programs for family caregivers of people with dementia, such as Savvy Caregiver, Staff Training in Assisted-Living Residences Caregivers, and Resources for Enhancing Alzheimer's Caregiver Health, have resulted in decreased agitation among people with dementia who live at home and reduced feelings of burden and depression among family caregivers.66⇓⇓–69
Nonpharmacologic interventions can be as simple as redirecting and refocusing the patient, increasing social interaction, initiating enjoyable activities, establishing regular sleep habits, eliminating sources of conflict and frustration (eg, activities that the patient can no longer undertake), and establishing rewards for successes, however small (Table 2).61 The principles of person-centered care, which aims to treat people as unique individuals with their own personality and preferences, are essential in the nonpharmacologic management of individuals with AD.17 For example, a person's religious background may influence his or her behavior. Patients of certain faiths may become agitated during intimate situations, such as bathing or dressing, when in the presence of caregivers who are of the opposite sex; a caregiver of the same sex may lead to improvement in behavior. The removal of any triggers of behavioral problems or the provision of comforting stimulation, such as the patient's favorite music, also may be beneficial.70
The use of behavioral interventions in dementia is hindered by the fact that the patient's cognitive functioning is declining progressively. As such, the effects of interventions must be monitored continually and adjustments made over time in response to new behaviors that may emerge.62 In patients with disruptive and hard-to-treat behavioral problems, referral to a behavioral specialist such as a geriatric psychiatrist should be considered.
Cognitive Behavioral Therapy
Over the past 10 years there has been an increasing interest in applying therapeutic frameworks, such as cognitive behavioral therapy (CBT), cognitive stimulation therapy (CST), and interpersonal therapy to dementia. These therapies are designed to actively stimulate and engage people with dementia; group therapy, such as that used for CST, provides an optimal learning environment and the social benefits of a group and aims to create an environment in which people learn and strengthen their existing resources. The principles of person-centered care are essential when delivering CST for individuals with dementia; as such, group members often are assigned a role within the group according to their interests and abilities. During each themed session, there is a range of activities available, which allows the facilitator to adapt the level of difficulty of the activities depending on the group's cognitive capabilities, interests, and gender mix; each individual can be provided with an activity suitable for him or her personally. Sessions for CST include physical games, sound and word association, and faces/scenes. Individuals are asked to give their opinions rather than provide factual answers, and multisensory stimulation is used when possible. Teri and Gallagher-Thompson71 reported positive findings from a clinical trial of CBT with individuals with early AD, and individual and group CBT also has been used with some favorable results.15,72 A CBT perspective is appropriate for people with dementia because many of the behavioral difficulties encountered emerge through one or more of the following cognitive features: cognitive misinterpretations, biases, distortions, erroneous problem-solving strategies, and communication difficulties. Put simply, many of the challenges posed by people with dementia are caused by their thinking style—the very thing that is addressed in CBT. CBT, therefore, offers a framework within which to understand the individual's distressing experiences, and this understanding allows the clinician to target interventions more appropriately.15
Pharmacologic Interventions
Pharmacologic interventions are necessary when nonpharmacologic strategies fail to reduce behavioral symptoms sufficiently. Patients treated with ChEIs, memantine, or both may also experience behavioral benefits in terms of reduced severity of existing behavioral disturbances and fewer new behavior symptoms29; usually agitation/aggression and irritability show responsiveness to ChEIs, memantine, or both, whereas depression, apathy, and anxiety do not.
If behavioral disturbances persist despite the use of ChEIs, memantine, or both, a psychotropic agent may be necessary.6 In accordance with the principles of geriatric psychiatry “start low and go slow, but go,” the psychotropic agent should be initiated in a low dosage and then increased slowly until an adequate response occurs or side effects emerge. After behavioral disturbances have been controlled for 4 to 6 months, the dosage of the psychotropic agent can be reduced periodically to determine whether continued pharmacotherapy is required. The choice of psychopharmacologic agent is determined by specific target symptoms; some behaviors, such as wandering and pacing, are not amenable to drug therapy. Medications used to treat behavioral disturbances and mood disorders are summarized in Table 3.6
Atypical Antipsychotics
Atypical antipsychotic drugs have been commonly used off-label in clinical practice for treatment of serious, dementia-associated agitation and aggression, although they are not approved by the FDA for such use. In addition, these agents have a black-box warning of increased mortality among elderly patients with dementia-related psychosis. A meta-analysis assessed the evidence for increased mortality from atypical antipsychotic drug treatment for people with dementia. Fifteen trials (9 unpublished), generally 10 to 12 weeks in duration and including 16 contrasts of atypical antipsychotic drugs with placebo, met criteria for inclusion (aripiprazole [n = 3], olanzapine [n = 5], quetiapine [n = 3], risperidone [n = 5]; one trial was counted both as a risperidone trial and an olanzapine trial). A total of 3353 patients were randomized to study drug and 1757 were randomized to placebo. Results demonstrated that atypical antipsychotics may be associated with a 50% increased risk of death from all causes, which is similar to older antipsychotics, but there was no obvious difference in risk between the 4 agents.73 In general, drugs may be used only when nonpharmacologic approaches have failed to control serious behavioral disruption adequately within 5 to 7 days.16 Members of a recent consensus conference, who are experts in the field of geriatric mental health, reviewed the available evidence regarding the safety and efficacy of antipsychotic drugs. They concluded that problems in clinical trial design may have contributed to the negative results reported and suggested that future studies be required to address the benefit–risk balance in this patient population.16 Nevertheless, the well-known incidence of side effects, such as sedation, falls, extrapyramidal signs, potential reduction in well-being and QoL,14 and even possible acceleration of cognitive decline,15,74,75 mean that the risk–benefit ratio must be considered carefully when prescribing these drugs to a generally frail population. If antipsychotics are indicated, then it is recommended that they are used at the lowest effective dose, with dosage reduced or treatment discontinuation considered on a regular basis.59
Agitation
Agitation and psychosis are distressing and are likely to overwhelm the caregiver's ability to cope. If behavioral and nonpharmacologic interventions are inadequate, mild agitation can be managed with low doses of medications, such as trazodone, carbamazepine, and valproate.59 Tricyclic antidepressants and benzodiazepines generally are avoided in this population.59 In patients with severe agitation and aggression, a recent consensus conference concluded that there is a need for an FDA-approved indication for treating dementia-related symptoms of severe and persistent or recurrent agitation and aggression, even in the absence of psychosis.16 Selective serotonin reuptake inhibitors seem to have efficacy for treatment of agitation in patients with AD. Studies have demonstrated benefits for agitation with citalopram compared with placebo76 and similar efficacy compared with risperidone.77
Apathy
Apathy as a distinct psychiatric syndrome is an evolving concept but generally has been defined as poor initiation, impaired persistence, indifference, reduced emotional response, and low social engagement.78 Although once believed to be just a symptom of depression, apathy is characterized primarily as a loss of motivation and reduced emotional reactivity, as opposed to depression, which is a mood disturbance.79 Based on a limited but increasing body of evidence, methylphenidate seems to have some efficacy for the treatment of apathy in older adults with AD.80
Depression
Depression is common in older adults, including those with AD, and often is undiagnosed and untreated. The efficacy of antidepressants in patients with AD who also suffer depression has been demonstrated in clinical trial; the most useful medications are those with minimal anticholinergic side effects. Selective serotonin reuptake inhibitors, such as citalopram (Celexa, Forest Laboratories, Inc.) and sertraline (Zoloft, Pfizer, New York, NY), seem to be effective and have fewer side effects compared with other antidepressants; as such, they are considered the agents of choice for the treatment of depression in patients with dementia, although direct head-to-head studies have yet to be undertaken.7,81
The Needs of the Caregiver
Caregivers can become exhausted and frustrated; suffer depression, anxiety, and health problems; and be at increased risk of death.2,9 Ideally, caregivers would receive assistance in caregiving, periodic assessment of their own health and welfare, support from family and friends, and respite care. One study has reported that the most consistently effective method of caregiver treatment interventions is to teach caregivers how to change or modify their interaction with the patient.30
Mittelman and colleagues82 have demonstrated the effectiveness of long-term behavioral interventions for caregivers. Caregivers of patients with AD often suffer from depression, and optimizing long-term social support (individual and family counseling, the continuous availability of ad hoc counseling, and support group participation) can have a significant impact on depression in caregivers.82 The same authors subsequently demonstrated that a program of counseling and support substantially increased the time spousal caregivers were able to care for AD patients at home. Patients whose spouses received the intervention experienced a 28% reduction in the rate of nursing home placement compared with usual care controls, with a difference in time to placement of 557 days. Improvements in caregivers' satisfaction with social support, response to patient behavior problems, and symptoms of depression collectively accounted for 61% of the intervention's beneficial impact on placement.83,84 Furthermore, these benefits were greatest in patients who had only mild dementia, when nursing home placement is generally least appropriate.83,84
In the event that insufficient resources are available to provide for and protect both patient and caregiver, nursing home placement needs to be considered.2 The progressive nature of dementia also must be emphasized, such that in the event of nursing home placement the caregiver does not consider it to be a failure on their part.2 Discussing the benefits and disadvantages of institutional care with caregivers is often challenging. Although consideration of the patient's previously expressed wishes is essential, caregivers often feel constrained by comments made years earlier and believe that the patient would not accept long-term care. It can be helpful to remind caregivers that earlier comments were made without a full appreciation of the current circumstances and that expectations almost always change with chronic illnesses.70
Future Therapies
ChEIs and memantine are symptomatic therapies that help maintain neuronal function but do not have a significant impact on the underlying disease process. Their benefits are mild, and treatments that modify the disease course are urgently needed.30,39 AD is the destruction of brain that cannot be regenerated, and any effective treatment needs to start before much brain is destroyed. There recently has been intense research interest in characterizing the earliest stages of AD that precede the crossing of the dementia threshold, defined by functional disability.85 Such preclinical disease detection may allow earlier therapeutic intervention before critical numbers of neurons are lost.
AD currently is thought to be a complex, multifactorial syndrome, unlikely to arise from a single causal factor; instead, a number of related biologic alterations are thought to contribute to its pathogenesis. In light of this, drug combinations that can act at different levels of the neurotoxic cascade offer new avenues toward curing AD and other neurodegenerative diseases.86 Effective treatment will require attacking multiple targets.87 At present, key therapeutic approaches include reduction of brain amyloid levels,30,88,89 prevention of τ hyperphosphorylation into intraneuronal neurofibrillary tangles,30,89 and stimulation of muscarinic acetylcholine receptors,90,91 although novel therapies increasingly are targeted to preserving energy metabolism in the mitochondria.92⇓–94
Conclusion
Family physicians play a crucial role in the care of patients with AD in terms of early detection, timely intervention, and effective ongoing management. Optimal management involves a multidimensional approach to treatment that includes the physician, geriatric care managers, social services, and the patient's family. The treatment of AD consists of both pharmacologic and nonpharmacologic interventions.
Nonpharmacologic interventions are recommended as the most appropriate initial strategy for managing problematic behaviors. Patients with AD function best in an environment that is safe, calm, and predictable. Interventions for improving behavior include reduction of environmental stressors and strategies to reduce the agitation and anxiety of the patient. These interventions can be as simple as redirecting and refocusing the patient, increasing social interaction, establishing regular sleep habits, eliminating sources of conflict and frustration, and establishing rewards for successes, however small. The role and needs of the caregiver are important, and the effectiveness of long-term behavioral interventions for caregivers has been demonstrated.
In the absence of means to reverse the pathologic processes of AD, the primary objectives of pharmacologic interventions are to preserve cognitive and functional ability, minimize behavioral disturbances, and slow disease progression. At present, four drugs are widely used to treat AD: 3 ChEIs, which are first-line treatment for patients with mild to moderate AD, and an NMDA antagonist approved for treatment of moderate to severe AD.
Acknowledgments
The authors would like to thank Frances Gambling for her editorial assistance with the manuscript. Administrative, editorial, and technical assistance was funded by Novartis Pharmaceuticals Corporation.
Notes
This article was externally peer reviewed.
Funding: Administrative, editorial, and technical assistance was funded by Novartis Pharmaceuticals Corporation.
Conflict of interest: none declared.
Disclosure: CHS has served as a consultant to Novartis Pharmaceuticals Corporation; has served as a speaker for Novartis Pharmaceuticals Corporation, Forest Pharmaceuticals, and Accera; and has received honoraria from both companies. JEG is a paid consultant for Pfizer, Eisai, Novartis Pharmaceuticals Corporation, Ortho-McNeil, and Forest. Neither author received financial support for this manuscript.
- Received for publication August 5, 2010.
- Revision received October 14, 2011.
- Accepted for publication November 14, 2011.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵